Abstract
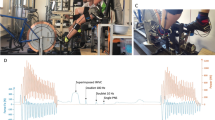
The purpose of this study was (1) to determine the relationship between each individual’s anaerobic power reserve (APR) [i.e., the difference between the maximum anaerobic (P ana) and aerobic power (P aer)] and fatigability during repeated-sprint exercise and (2) to examine the acute effects of repeated sprints on neuromuscular activity, as evidenced by changes in the surface electromyogram (EMG) signals. Eight healthy males carried out tests to determine P ana (defined as the highest power output attained during a 6-s cycling sprint), P aer (defined as the highest power output achieved during a progressive, discontinuous cycling test to failure) and a repeated cycling sprint test (10 × 6-s max sprints with 30 s rest). Peak power output (PPO) and mean power output (MPO) were calculated for each maximal 6-s cycling bout. Root mean square (RMS) was utilized to quantify EMG activity from the vastus lateralis (VL) muscle of the right leg. Over the ten sprints, PPO and MPO decreased by 24.6 and 28.3% from the maximal value (i.e., sprint 1), respectively. Fatigue index during repeated sprints was significantly correlated with APR (R = 0.87; P < 0.05). RMS values decreased over the ten sprints by 14.6% (±6.3%). There was a strong linear relationship (R 2 = 0.97; P < 0.05) between the changes in MPO and EMG RMS from the vastus lateralis muscle during the ten sprints. The individual advantage in fatigue-resistance when performing a repeated sprint task was related with a lower anaerobic power reserve. Additionally, a suboptimal net motor unit activity might also impair the ability to repeatedly generate maximum power outputs.





Similar content being viewed by others
References
Akima H, Kinugasa R, Kuno S (2004) Recruitment of the thigh muscles during sprint cycling by muscle functional magnetic resonance imaging. Int J Sports Med 25:1–8
Balsom P, Ekblom B, Sjodin B (1994a) Enhanced oxygen availablility during high intensity intermittent exercise decreases anaerobic metabolite concentration in blood. Acta Physiol Scand 150:455–456
Balsom PD, Gaitanos GC, Ekblom B, Sjodin B (1994b) Reduced oxygen availability during high intensity intermittent exercise impairs performance. Acta Physiol Scand 152:279–285
Barclay CJ (1996) Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J Physiol 497:781–794
Bellemare F, Garzaniti N (1988) Failure of neuromuscular propagation during human maximal voluntary contraction. J Appl Physiol 64:1084–1093
Billaut F, Giacomoni M, Falgairette G (2003) Maximal intermittent exercise: effects of recovery duration and gender. J Appl Physiol 95:1632–1637
Billaut F, Basset FA, Falgairette G (2005). Muscle coordination changes during intermittent cycling sprints. Neurosci Lett 380:265–269
Billaut F, Basset FA, Giacomoni M, Lemaître F, Tricot V, Falgairette G (2006) Effect of high-intensity intermittent cycling sprints on neuromuscular activity. Int J Sports Med 27:25–30
Bishop D, Edge J (2006) Determinants of repeated-sprint ability in females matched for single-sprint performance. Eur J Appl Physiol 97:373–379
Bishop D, Spencer M (2004) Determinants of repeated sprint ability in well-trained team-sport and endurance-trained athletes. J Sports Med Phys Fit 44:1–6
Bishop D, Lawrence S, Spencer M (2003) Predictors of repeated-sprint ability in elite female hockey players. J Sci Med Sport 6:199–209
Bishop D, Davis C, Edge J, Goodman C (2004a) Induced metabolic alkalosis effects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc 36:807–813
Bishop D, Edge J, Goodman C (2004b) The relationship between muscle buffer capacity and repeated-sprint ability in females. Eur J Appl Physiol 92:540–547
Bundle MW, Hoyt RW, Weyand PG (2003) High-speed running performance: a new approach to assessment and prediction. J Appl Physiol 95:1955–1962
Drust B, Rasmussen P, Mohr M, Nielsen B, Nybo L (2005) Elevations in core and muscle temperature impairs repeated sprint performance. Acta Physiol Scand 183:181–190
Dupont G, Millet GP, Guinhouya C, Berthoin S (2005) Relationship between oxygen uptake kinetics and performance in repeated running sprints. Eur J Appl Physiol 95: 27–34
Enoka RM, Stuart DG (1992) Neurobiology of muscle fatigue. J Appl Physiol 72:1631–1648
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96:1486–1495
Ferrauti A, Pluim BM, Weber K (2001) The effect of recovery duration on running speed and stroke quality during intermittent training drills in elite tennis players. J Sports Sci 19:235–242
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75:712–719
Gerdle B, Karlsson S, Crenshaw AG, Elert J, Fridén J (2001) The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur J Appl Physiol 81:2–10
Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA (2003) Interaction of fibre type, potentiation and fatigue in human extensor muscles. Acta Physiol Scand 178:165–173
Hamilton AL, Nevill ME, Brooks S, Williams C (1991) Physiological responses to maximal intermittent exercise: differences between endurance-trained runners and games players. J Sports Sci 9:371–382
Hautier CA, Arsac LM, Deghdegh K, Souquet J, Belli A, Lacour J-R (2000) Influence of fatigue on EMG/force ration and cocontraction in cycling. Med Sci Sports Exerc 32:839–843
Hirvonen J, Rehunen S, Rusko H, Harkonen M (1987) Breakdown of high-energy phosphate compounds and lactate accumulation during short supramaximal exercise. Eur J Appl Physiol 56:253–259
Hoylmard DJ, Cheetham ME, Lakomy HKA, Williams C (1987) Effects of recovery duration on performance during multiple treadmill sprints. In: Reilly T, Lees A, Davids K, Murphy WJ (eds) Science and football. E & F.N. Spoon, London
Hunter SK, Enoka RM (2001) Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 91:2686–2694
James C, Sacco P, Jones DA (1995) Loss of power during fatigue of human leg muscles. J Physiol 484:237–246
Karatzaferi C, de Haan A, van Mechelen W, Sargeant AJ (2001) Metabolism changes in single human fibres during brief maximal exercise. Exp Physiol 86:411–415
Kawakami Y, Amemiya K, Kanehisa H, Ikegawa S, Fukunaga T (2000) Fatigue responses of human triceps surae muscles during repetitive maximal isometric contractions. J Appl Physiol 88:1969–1975
Kinugasa R, Akima H, Ota A, Ohta A, Sugiura K, Kuno S (2004) Short-term creatine supplementation does not improve muscle activation or sprint performance in humans. Eur J Appl Physiol 91:230–237
Krustrup P, Soderlund K, Mohr M, Gonzalez-Alonso J, Bangsbo J (2004) Recruitment of fibre types and quadriceps muscle portions during repeated, intense knee-extensor exercise in humans. Pflugers Arch 449:56–65
McMahon S, Wenger HA (1998) The relationship between aerobic fitness and both power output and subsequent recovery during maximal intermittent exercise. J Sci Med Sport 1:219–227
Mendez-Villanueva A, Bishop D, Hamer P (2007a) Fatigue responses during repeated sprints matched for initial mechanical output. Med Sci Sports Exerc 39:2219–225
Mendez-Villanueva A, Bishop D, Hamer P (2007b) Reproducibility of a 6-s maximal cycling sprint test. J Sci Med Sport 10:323–326
Nordlund MM, Thorstensson A, Cresswell AG (2004) Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol 96:218–225
Racinais S, Bishop D, Denis R, Lattier G, Mendez-Villanueva A, Perrey S (2007) Muscle deoxygenation and neural drive to the muscle during repeated sprint cycling. Med Sci Sports Exerc 39:268–274
Rome LC, Lindstedt SL (1998) The quest for speed: muscles built for high-frequency contractions. News Physiol Sci 13:261–268
Taylor JL, Allen GM, Butler JE, Gandevia SC (2000) Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol 89:305–313
Thomas C, Sirvent P, Perrey S, Raynaud E, Mercier J (2004) Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J Appl Physiol 97:2132–2138
Tomlin DL, Wenger HA (2002) The relationship between aerobic fitness, power maintenance and oxygen consumption during intense intermittent exercise. J Sci Med Sport 5:194–203
Wadley G, Le Rossignol P (1998) The relationship between repeated sprint ability and the aerobic and anaerobic energy systems. J Sci Med Sport 1:100–110
Westerblad H, Allen DG, Lannergren J (2002) Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci 17:17–21
Weyand PG, Bundle MW (2005) Energetics of high-speed running: integrating classical theory and contemporary observations. Am J Physiol 288:R956–R965
Weyand PG, Lin JE, Bundle MW (2006) Sprint performance–duration relationships are set by the fractional duration of external force application. Am J Physiol 290:R758–R765
Zhang S, Bruton JD, Katz A, Westerblad H (2006) Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol 572:551–559
Acknowledgments
This study was supported by a grant from the Gatorade Sports Science Institute. The authors wish to express their sincere gratitude to all the participants for their maximal effort and cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendez-Villanueva, A., Hamer, P. & Bishop, D. Fatigue in repeated-sprint exercise is related to muscle power factors and reduced neuromuscular activity. Eur J Appl Physiol 103, 411–419 (2008). https://doi.org/10.1007/s00421-008-0723-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0723-9