Abstract
Purpose
To report the long-term results of anti-vascular endothelial growth factor (VEGF) therapy for choroidal neovascularization (CNV) secondary to pathological myopia (PM).
Methods
Prospective interventional study with extension phase. Eyes affected by CNV due to PM included. All patients received an intravitreal bevacizumab injection (1.25 mg/0.05 ml) at baseline. Re-treatment was considered at each follow-up visit.
Results
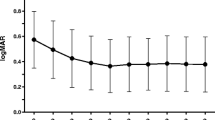
The study included 101 consecutive eyes of 86 patients. All patients reached 24 months of follow-up. After 24 months, mean best-corrected visual acuity (BCVA) improvement was −0.13 (95 % CI: −0.2; −0.05) logMAR (p < 0.001) and central retinal thickness (CRT) decreased on average by 67 (95 % CI: 27; 102) μm (p < 0.01). The chorioretinal atrophy (CRA) area increased significantly after 2 years of follow-up (+7.82 mm2, p < 0.0001). Patients received 4.1 treatments, on average. Thirty-two eyes were included in the extension phase (from 24 to 60 months of follow-up). Visual acuity improved on average by −0.05 (95 % CI: −0.2; 0.1) logMAR (p > 0.05) compared to baseline. Mean reduction in CRT was 102 (95 % CI: 64;141) μm (p < 0.01). The CRA area enlarged significantly after 5 years of follow-up (+14.15 mm2, p < 0.0001). Patients received a mean of 6.7 treatments in 60 months.
Conclusions
An individualized regimen with intravitreal bevacizumab to treat CNV secondary to PM resulted in BCVA improvement and CRT decrease at 2 and 5 years. The main visual benefit was obtained between month 3 and month 24. A gradual loss of the initial BCVA gain was observed starting from month 30 to month 60 due to progression of CRA.









Similar content being viewed by others
References
Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, Tokoro T, Mochizuki M (2003) Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 110:1297–1305
Wong TY, Ohno-Matsui K, Leveziel N, Holz FG, Lai TY, Yu HG, Lanzetta P, Chen Y, Tufail A (2014) Myopic choroidal neovascularization: current concepts and update on clinical management. Br J Ophthalmol. doi:10.1136/bjophthalmol-2014-305131
Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P (2014) Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 157:9–25
Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL (1984) Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 91:1573–1581
Pece A, Brancato R, Avanza P, Camesasca F, Galli L (1994) Laser photocoagulation of choroidal neovascularization in pathologic myopia: long-term results. Int Ophthalmol 18:339–344
Virgili G, Menchini F (2005) Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst Rev 4:CD004765
Verteporfin in Photodynamic Therapy Study Group (2001) Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial-VIP report No. 1. Ophthalmology 108:841–852
Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, Lim JI, Menchini U, Miller JW, Mones JM, Potter MJ, Pournaras C, Reaves A, Rosenfeld P, Schachat AP, Schmidt-Erfurth U, Sickenberg M, Singerman LJ, Slakter JS, Strong HA, Virgili G, Williams GA (2003) Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial-VIP report no. 3. Ophthalmology 110:667–673
Gharbiya M, Giustolisi R, Allievi F, Fantozzi N, Mazzeo L, Scavella V, Gabrieli CB (2010) Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab—a randomized controlled trial. Am J Ophthalmol 149:458–464
Baba T, Kubota-Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S (2010) Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularisation. Br J Ophthalmol 94:864–870
Franqueira N, Cachulo ML, Pires I, Fonseca P, Marques I, Figueira J, Silva R (2012) Long-term follow-up of myopic choroidal neovascularization treated with ranibizumab. Ophthalmologica 227:39–44
Lai TY, Luk FO, Lee GK, Lam DS (2012) Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye 26:1004–1011
Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, Stemper B, Asmus F, Zeitz O, Ishibashi T, MYRROR Investigators (2015) Intravitreal Aflibercept Injection in Patients with Myopic Choroidal Neovascularization: The MYRROR Study. Ophthalmology 122:1220-1227
Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, Wong TY, Silva R, Pilz S, Gekkieva M, RADIANCE Study Group (2014) RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 121:682–692
Tufail A, Narendran N, Patel PJ, Sivaprasad S, Amoaku W, Browning AC, Osoba O, Gale R, George S, Lotery AJ, Majid M, McKibbin M, Menon G, Andrews C, Brittain C, Osborne A, Yang Y (2013) Ranibizumab in myopic choroidal neovascularization: The 12-month results from the REPAIR study. Ophthalmology 120:1944–1945
Hamelin N, Glacet-Bernard A, Brindeau C, Mimoun G, Coscas G, Soubrane G (2002) Surgical treatment of subfoveal neovascularization in myopia: Macular translocation versus surgical removal. Am J Ophthalmol 133:530–536
Ruiz-Moreno JM, de la Vega C (2001) Surgical removal of subfoveal choroidal neovascularization in highly myopic patients. Br J Ophthalmol 85:1041–1043
Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS (2007) Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathological myopia. Br J Ophthalmol 91:157–160
Chan WM, Lai TY, Liu DT, Lam DS (2007) Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology 114:2190–2196
Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB (2009) Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol 147:84–93
Monés JM, Amselem L, Serrano A, Garcia M, Hijano M (2009) Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye 23:1275–1280
Silva RM, Ruiz-Moreno JM, Rosa P, Carneiro A, Nascimento J, Rito LF, Cachulo ML, Carvalheira F, Murta JN (2010) Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina 30:407–412
Ikuno Y, Nagai Y, Matsuda S, Arisawa A, Sho K, Oshita T, Takahashi K, Uchihori Y, Gomi F (2010) Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol 149:140–146
Ruiz-Moreno JM, Montero JA (2010) Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol 248:937–941
Gharbiya M, Cruciani F, Parisi F, Cuozzo G, Altimari S, Abdolrahimzadeh S (2012) Long-term results of intravitreal bevacizumab for choroidal neovascularisation in pathological myopia. Br J Ophthalmol 96:1068–1072
Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Bandello F (2011) Intravitreal bevacizumab therapy on an as-per-needed basis in subfoveal choroidal neovascularization secondary to pathological myopia: 2-year outcomes of a prospective case series. Retina 31:1841–1847
Peiretti E, Vinci M, Fossarello M (2012) Intravitreal bevacizumab as a treatment for choroidal neovascularization secondary to myopia:4-year study results. Can J Ophthalmol 47:28–33
Oishi A, Yamashiro K, Tsujikawa A, Ooto S, Tamura H, Nakata I, Miyake M, Yoshimura N (2013) Long-term effect of intravitreal injection of anti-VEGF agent for visual acuity and chorioretinal atrophy progression in myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 251:1–7
Yodoi Y, Tsujikawa A, Nakanishi H, Otani A, Tamura H, Ojima Y, Hayashi H, Yoshimura N (2009) Central retinal sensitivity after intravitreal injection of bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol 147:816–824
Uemoto R, Nakasato-Sonn H, Kawagoe T, Akira M, Okada E, Mizuki N (2012) Factors associated with enlargement of chorioretinal atrophy after intravitreal bevacizumab for myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 250:989–997
Khanifar AA, Lederer DE, Ghodasra JH, Stinnett SS, Lee JJ, Cousins SW, Bearelly S (2012) Comparison of color fundus photographs and fundus autofluorescence images in measuring geographic atrophy area. Retina 32:1884–1891
Ruiz-Moreno JM, Montero JA, Amat-Peral P (2011) Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses. Graefes Arch Clin Exp Ophthalmol 249:595–599
Ruiz-Moreno JM, Arias L, Montero JA, Carneiro A, Silva R (2013) Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol 97:1447–1450
CATT Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Two years results. Ophthalmology 119:1388–1398
Acknowledgments
No funding support was received for this work.
Conflict of interest
V. Sarao, D.Veritti and S. Macor declare that they have no conflict of interest in the subject matter or materials discussed in this manuscript. P.Lanzetta has acted as a consultant for Alcon, Alimera, Allergan, Bausch & Lomb, Bayer, C, Novartis, Roche and Teva.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarao, V., Veritti, D., Macor, S. et al. Intravitreal bevacizumab for choroidal neovascularization due to pathologic myopia: long-term outcomes. Graefes Arch Clin Exp Ophthalmol 254, 445–454 (2016). https://doi.org/10.1007/s00417-015-3076-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3076-1