Abstract
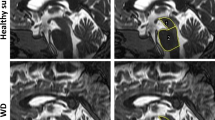
Small vessel cerebrovascular disease (SVCD) is one of the most frequent vessel disorders in the aged brain. Among the spectrum of neurological disturbances related to SVCD, oculomotor dysfunction is a not well understood symptom- in particular, it remains unclear whether vascular lesion load in specific brain regions affects oculomotor function independent of cognitive decline in SVCD patients or whether the effect of higher brain function deficits prevails. In this study, we examined a cohort of 25 SVCD patients and 19 healthy controls using video-oculographic eye movement recording in a laboratory environment, computer-based MRI assessment of white matter lesion load (WMLL), assessment of extrapyramidal motor deficits, and psychometric testing. In comparison to controls, the mean WMLL of patients was significantly larger than in controls. With respect to eye movement control, patients performed significantly worse than controls in almost all aspects of oculomotion. Likewise, patients showed a significantly worse performance in all but one of the neuropsychological tests. Oculomotor deficits in SVCD correlated with the patients’ cognitive dysfunctioning while there was only weak evidence for a direct effect of WMLL on eye movement control. In conclusion, oculomotor impairment in SVCD seems to be mainly contingent upon cognitive deterioration in SVCD while WMLL might have only a minor specific effect upon oculomotor pathways.




Similar content being viewed by others
References
Behrouz R, Malek AR, Torbey MT (2012) Small vessel cerebrovascular disease: the past, present, and future. Stroke Res Treat 2012:839151. doi:10.1155/2012/839151
Grinberg LT, Thal DR (2010) Vascular pathology in the aged human brain. Acta Neuropathol 119:277–290. doi:10.1007/s00401-010-0652-7
Jellinger KA (2008) The pathology of “vascular dementia”: a critical update. J Alzheimer’s Dis: JAD 14:107–123
van Norden AG, de Laat KF, Gons RA et al (2011) Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol 11:29. doi:10.1186/1471-2377-11-29
Inzitari D, Pracucci G, Poggesi A et al (2009) Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 339:b2477
Patel B, Markus HS (2011) Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke: Off J Int Stroke Soc 6:47–59. doi:10.1111/j.1747-4949.2010.00552.x
Rost NS, Rahman RM, Biffi A et al (2010) White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 75:1670–1677. doi:10.1212/WNL.0b013e3181fc279a
Fazekas F, Barkhof F, Wahlund LO et al (2002) CT and MRI rating of white matter lesions. Cerebrovasc Dis (Basel, Switzerland) 13(Suppl 2):31–36
Wright CB, Paik MC, Brown TR et al (2005) Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke J Cereb Circ 36:1207–1211. doi:10.1161/01.STR.0000165923.02318.22
Rosler A, Billino J, Muller NG et al (2005) Visual search in patients with subcortical vascular dementia: short fixations but long reaction times. Dement Geriatr Cogn Disord 20:375–380. doi:10.1159/000089104
Pinkhardt EH, Kassubek J, Süssmuth S et al (2009) Comparison of smooth pursuit eye movement deficits in multiple system atrophy and Parkinson’s disease. J Neurol 256:1438–1446. doi:10.1007/s00415-009-5131-5
Tinetti ME, Williams TF, Mayewski R (1986) Fall risk index for elderly patients based on number of chronic disabilities. Am J Med 80(3):429–434
Müller HP, Unrath A, Ludolph AC, Kassubek J (2007) Preservation of diffusion tensor properties during spatial normalization by use of tensor imaging and fibre tracking on a normal brain database. Phys Med Biol 52:N99–N109. doi:10.1088/0031-9155/52/6/N01
Eritaia J, Wood SJ, Stuart GW et al (2000) An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med 44:973–977
Reite M, Reite E, Collins D et al (2010) Brain size and brain/intracranial volume ratio in major mental illness. BMC Psychiatry 10:79. doi:10.1186/1471-244X-10-79
Morris JC, Edland S, Clark C et al (1993) The consortium to establish a registry for Alzheimer“s disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer”s disease. Neurology 43:2457–2465
Lewandowski LJ (1984) The Symbol Digits Modalities Test: a screening for brain-damaged children. Percept Mot Skills 59:615–618
Jensen AR, Rohwer WD (1966) The Stroop color-word test: a review. Acta Psychol (Amst) 25:36–93
Scheurich A, Fellgiebel A, Schermuly I et al (2008) Experimental evidence for a motivational origin of cognitive impairment in major depression. Psychol Med 38:237–246. doi:10.1017/S0033291707002206
Regard M, Strauss E, Knapp P (1982) Children’s production on verbal and non-verbal fluency tasks. Percept Mot Skills 55:839–844
Rujescu D, Meisenzahl EM, Krejcova S, et al. (2007) Plexin B3 is genetically associated with verbal performance and white matter volume in human brain. Mol Psychiatry 12:190–4, 115. doi: 10.1038/sj.mp.4001903
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Hooper HE (1958) The Hooper Visual Organisation Test: Manual. Western Psychological Services, Los Angeles
Roberts RE, Vernon SW (1983) The Center for Epidemiological Studies Depression Scale: its use in a community sample. Am J Psychiatry 140(1):41–46
Nopoulos P, Flaum M, O’Leary D, Andreasen NC (2000) Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res 98:1–13
Pantoni L, Simoni M, Pracucci G et al (2002) Visual Rating Scales for age-related white matter changes (leukoaraiosis): can the heterogeneity be reduced? Stroke J Cereb Circ 33:2827–2833. doi:10.1161/01.str.0000038424.70926.5e
Marchant NL, Reed BR, Sanossian N et al (2013) The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol 1:8. doi:10.1001/2013.jamaneurol.405
Bunce D, Anstey KJ, Cherbuin N et al (2010) Cognitive deficits are associated with frontal and temporal lobe white matter lesions in middle-aged adults living in the community. PLoS One 5:e13567. doi:10.1371/journal.pone.0013567
Mueller SG, Mack WJ, Mungas D et al (2010) Influences of lobar gray matter and white matter lesion load on cognition and mood. Psychiatry Res 181:90–96. doi:10.1016/j.pscychresns.2009.08.002
Culang-Reinlieb ME, Johnert LC, Brickman AM et al (2010) MRI-defined vascular depression: a review of the construct. Int J Geriatr Psychiatry. doi:10.1002/gps.2668
Brown FW, Lewine RJ, Hudgins PA, Risch SC (1992) White matter hyperintensity signals in psychiatric and nonpsychiatric subjects. Am J Psychiatry 149:620–625
Dubin MJ, Dubin M, Weissman MM et al (2012) Identification of a circuit-based endophenotype for familial depression. Psychiatry Res 201:175–181. doi:10.1016/j.pscychresns.2011.11.007
Chan F, Armstrong IT, Pari G et al (2005) Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia 43:784–796. doi:10.1016/j.neuropsychologia.2004.06.026
Hood AJ, Amador SC, Cain AE et al (2007) Levodopa slows prosaccades and improves antisaccades: an eye movement study in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78:565–570. doi:10.1136/jnnp.2006.099754
Pinkhardt EH, Jürgens R, Lulé D et al (2012) Eye movement impairments in Parkinson’s disease: possible role of extradopaminergic mechanisms. BMC Neurol 12:5. doi:10.1186/1471-2377-12-5
MacAskill MR, Graham CF, Pitcher TL et al (2012) The influence of motor and cognitive impairment upon visually-guided saccades in Parkinson’s disease. Neuropsychologia 50:3338–3347. doi:10.1016/j.neuropsychologia.2012.09.025
Pinkhardt EH, Jürgens R, Becker W et al (2008) Differential diagnostic value of eye movement recording in PSP-parkinsonism, Richardson“s syndrome, and idiopathic Parkinson”s disease. J Neurol 255:1916–1925. doi:10.1007/s00415-009-0027-y
Leigh RJ, Zee DS (2006) The neurology of eye movement, 4th edn. Oxford University Press, Oxford
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978
DeLong MR, Wichmann T (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64:20–24. doi:10.1001/archneur.64.1.20
Garbutt S, Matlin A, Hellmuth J et al (2008) Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain: J Neurol 131:1268–1281. doi:10.1093/brain/awn047
Meyniel C, Rivaud-Pechoux S, Damier P, Gaymard B (2005) Saccade impairments in patients with fronto-temporal dementia. J Neurol Neurosurg Psychiatry 76:1581–1584. doi:10.1136/jnnp.2004.060392
Donaghy CC, Thurtell MJM, Pioro EPE et al (2010) Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. J Neurol Neurosurg Psychiatry 82:110–116. doi:10.1136/jnnp.2010.212407
McCall WV, Dunn AG (2003) Cognitive deficits are associated with functional impairment in severely depressed patients. Psychiatry Res 121:179–184
Porter RJ, Gallagher P, Thompson JM, Young AH (2003) Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 182:214–220
Schmidt R, Fazekas F, Offenbacher H et al (1993) Neuropsychologic correlates of MRI white matter hyperintensities: a study of 150 normal volunteers. Neurology 43:2490–2494
Launer LJ, Hughes TM, White LR (2011) Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 70:774–780. doi:10.1002/ana.22520
Smallwood A, Oulhaj A, Joachim C et al (2012) Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol 38:337–343. doi:10.1111/j.1365-2990.2011.01221.x
van Norden AGW, de Laat KF, van Dijk EJ et al (2012) Diffusion tensor imaging and cognition in cerebral small vessel disease: the RUN DMC study. Biochim Biophys Acta 1822:401–407. doi:10.1016/j.bbadis.2011.04.008
De Groot JC, de Leeuw F-E, Oudkerk M et al (2002) Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 52:335–341. doi:10.1002/ana.10294
Meier IB, Manly JJ, Provenzano FA et al (2012) White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc: JINS 18:414–427. doi:10.1017/S1355617712000227
van Norden AGW, van Uden IWM, de Laat KF et al (2012) Cognitive function in small vessel disease: the additional value of diffusion tensor imaging to conventional magnetic resonance imaging: the RUN DMC study. J Alzheimer’s Dis: JAD 32:667–676. doi:10.3233/JAD-2012-120784
Conflicts of interest
There are no conflicts of interest to report for all authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. H. Pinkhardt and H. Issa contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2014_7275_MOESM1_ESM.tiff
Supplementary Fig. 1. Pattern of oculomotor differences between SVCD patients (red) and controls (blue); median values with 90 % ranges (black bars). Note different scalings depending on parameter type (cf. Table 2 for exact values). Significant differences marked by * (p < 0.01) or ** (p < 0.001). SacGn, saccade gain with target steps of 20°; SacPV, peak velocity of 20°-saccades; SacLat, latency of saccades; RAVS, number of rapidly alternating voluntary back and forth saccades between two fixed targets at 10° left and right within 30 s; HdGn, gain of head component of head-free gaze saccades elicited by target steps of 30°; HdPV, peak velocity of 30° head movements during head-free gaze saccades; SPM1 and SPM2, gain of sinusoidal smooth pursuit eye movements at 0.125 and 0.375 Hz, respectively; DelErr and AntErr, percent errors in the delayed and anti-saccade tasks. Saccade and SPEM measures represent averages of horizontal and vertical movements (TIFF 4,004 kb)
Rights and permissions
About this article
Cite this article
Pinkhardt, E.H., Issa, H., Gorges, M. et al. Do eye movement impairments in patients with small vessel cerebrovascular disease depend on lesion load or on cognitive deficits? A video-oculographic and MRI study. J Neurol 261, 791–803 (2014). https://doi.org/10.1007/s00415-014-7275-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7275-1