Abstract
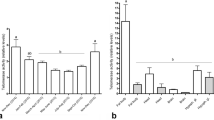
Telomerase is an enzyme that adds repeats of DNA sequences to the ends of chromosomes, thereby preventing their shortening. Telomerase activity is associated with proliferative status of cells, organismal development, and aging. We report an analysis of telomerase activity and telomere length in the honeybee, Apis mellifera. Telomerase activity was found to be regulated in a development and caste-specific manner. During the development of somatic tissues of larval drones and workers, telomerase activity declined to 10 % of its level in embryos and remained low during pupal and adult stages but was upregulated in testes of late pupae, where it reached 70 % of the embryo level. Upregulation of telomerase activity was observed in the ovaries of late pupal queens, reaching 160 % of the level in embryos. Compared to workers and drones, queens displayed higher levels of telomerase activity. In the third larval instar of queens, telomerase activity reached the embryo level, and an enormous increase was observed in adult brains of queens, showing a 70-fold increase compared to a brain of an adult worker. Southern hybridization of terminal TTAGG fragments revealed a high variability of telomeric length between different individuals, although the same pattern of hybridization signals was observed in different tissues of each individual.



Similar content being viewed by others
References
Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88:557–579
Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA (2012) Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 4:691–704
Cawthon R, Smith K, O’Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Cong Y, Wright WE, Shay JW (2002) Human telomerase and its regulation. Microbiol Mol Biol Rev 66:407–425
Dixon L, Kuster R, Rueppell O (2014) Reproduction, social behavior, and aging trajectories in honeybee workers. Age 36:89–101
Ferrón S, Mira H, Franco S, Cano-Jaimez M, Bellmunt E, Ramírez C, Fariñas I, Blasco MA (2004) Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development 131:4059–4070
Ferrón SR, Marqués-Torrejón MA, Mira H, Flores I, Taylor K, Blasco MA, Fariñas I (2009) Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci 29:14394–14407
Flanders SE (1960) Caste in the honey bee. Insectes Soc 7:9–16
Flores I, Benetti R, Blasco MA (2006) Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol 18:254–260
Fossel M (2012) Use of telomere length as a biomarker for aging and Age-related disease. Curr Transl Geriatr Exp Gerontol Rep 1:121–127
Frydrychova R, Grossmann P, Trubac P, Vitkova M, Marec F (2004) Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome 47:163–178
Frydrychova R, Marec F (2002) Repeated losses of TTAGG telomere repeats in evolution of beetles (Coleoptera). Genetica 115:179–187
Göhring J, Fulcher N, Jacak J, Riha K (2014) TeloTool: a new tool for telomere length measurement from terminal restriction fragment analysis with improved probe intensity correction. Nucleic Acids Res 42:1–10
Gomes NMV, Shay JW, Wright WE (2011) Telomere biology in metazoa. Fed Eur Biochem Soc 584:3741–3751
Greider CW (1996) Telomere length regulation. Annu Rev Biochem 65:337–365
Harshman LG, Zera AJ (2007) The cost of reproduction: the devil in the details. Trends Ecol Evol 22:80–86
Hsieh YS, Hsu CY (2011) The changes of age-related molecules in the trophocytes and fat cells of queen honeybees (Apis mellifera). Apidologie 42:728–739
Jiang H, Ju Z, Rudolph KL (2007) Telomere shortening and ageing. Z Gerontol Geriatr 40:314–324
Keller L, Jemielity S (2006) Social insects as a model to study the molecular basis of ageing. Exp Gerontol 41:553–556
Korandová M, Krůček T, Vrbová K, Frydrychová RC (2014) Distribution of TTAGG- specific telomerase activity in insects. Chromosome Res 22:495–503
Lin J, Epel E, Blackburn E (2012) Telomeres and lifestyle factors: roles in cellular aging. Mutat Res 730:85–89
Mason JM, Randall TA, Capkova Frydrychova R (2015) Telomerase lost? Chromosoma. doi:10.1007/s00412-015-0528-7
Nakamura KI, Izumiyama-Shimomura N, Sawabe M, Arai T, Aoyagi Y, Fujiwara M, Tsuchiya E, Kobayashi Y, Kato M, Oshimura M, Sasajima K, Nakachi K, Takubo K (2002) Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J Invest Dermatol 119:1014–1019
Page RE, Peng CY-S (2001) Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol 36:695–711
Patrício K, Cruz-Landim C (2002) Mating influence in the ovary differentiation in adult queens of Apis mellifera L. (Hymenoptera, Apidae). Braz J Biol 62:641–649
Pfeiffer KJ, Crailsheim K (1998) Drifting of honeybees. Insectes Soc 45:151–167
Remolina SC, Hughes KA (2008) Evolution and mechanisms of long life and high fertility in queen honey bees. Age 30:177–185
Reznick D (1985) Costs of reproduction: an evaluation of the empirical evidence. Oikos 44:257–267
Robertson HM, Gordon KHJ (2006) Canonical TTAGG-repeat telomeres and telomerase in the honey bee, Apis mellifera. Genome Res 16:1345–1351
Rolyan H, Scheffold A, Heinrich A, Begus-Nahrmann Y, Langkopf BH, Hölter SM, Vogt-Weisenhorn DM, Liss B, Wurst W, Lie DC, Thal DR, Biber K, Rudolph KL (2011) Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain 134:2044–2056
Sahara K, Marec F, Traut W (1999) TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res 7:449–460
Sasaki T, Fujiwara H (2000) Detection and distribution patterns of telomerase activity in insects. Eur J Biochem 267:3025–3031
Shay JW, Wright WE (2000) Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 1:72–76
Starkweather A, Alhaeeri A, Montpetit A, Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE, Jackson-Cook CK (2014) An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurse Res 63:36–50
Stindl R, Stindl W (2010) Vanishing honey bees: is the dying of adult worker bees a consequence of short telomeres and premature aging? Med Hypotheses 75:387–390
Tan TCJ, Rahman R, Jaber-Hijazi F, Felix DA, Chen C, Louis EJ, Aboobaker A (2012) Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms. Proc Natl Acad Sci U S A 109:4209–4214
vanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, Frazier M, Frazier J, Cox-Foster D, Chen Y, Underwood R, Tarpy DR, Pettis JS (2009) Colony collapse disorder: a descriptive study. PloS One 4(8):e6481
vanEngelsdorp D, Meixner MD (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103(suppl 1):S80–S95
Venkatesan RN, Price C (1998) Telomerase expression in chickens: constitutive activity in somatic tissues and down-regulation in culture. Proc Natl Acad Sci U S A 95:14763–14768
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW (1996) Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18:173–179
Wyatt HDM, West SC, Beattie TL (2010) InTERTpreting telomerase structure and function. Nucleic Acids Res 38:5609–5622
Acknowledgments
We are grateful to James Mason for critical reading of the manuscript. We thank Dalibor Titěra, Denisa Pánková, Ivana Bendová, Jan Benda, and Václav Doubek for allowing us to collect honeybee samples. We thank Marie Korchová for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Grant No. 14-07172S from the Grant Agency of the Czech Republic, by the Grant No. 052/2013/P from the Grant Agency of the University of South Bohemia, and by additional grants from the Grant Agency of the University of South Bohemia. We acknowledge the use of research infrastructure that has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 316304.
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Detection of telomerase activity using a polyacrylamide gel. The TRAP assay was performed in protein extract of embryos (extract 1) and head of adult worker (extract 2). The TRAP products were run in a 15 % polyacrylamide gel and stained with SYBR-Green I. Samples treated with RNase A were used as negative controls. (TIFF 695 kb)
Rights and permissions
About this article
Cite this article
Korandová, M., Frydrychová, R.Č. Activity of telomerase and telomeric length in Apis mellifera . Chromosoma 125, 405–411 (2016). https://doi.org/10.1007/s00412-015-0547-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-015-0547-4