Abstract
A general method for calculating doses absorbed from isotopes released in nuclear accidents is presented. As an example, this method was used to calculate doses for inhabitants of Southern Poland due to inhalation of 131I released due to the Fukushima nuclear plant accident. 131I activity measurements in the air of that region provided the basis for the study. The proposed model is based on a complex biokinetic model for iodine merging the Leggett model developed in 2010 with the human respiratory tract and gastrointestinal tract models recommended by the International Commission on Radiological Protection (ICRP). This model is described here, and it is demonstrated that resulting dose estimates are consistent with those obtained using the ICRP methodology. Using the developed model, total doses were calculated for six age groups of both genders, for gaseous and aerosol fractions alike. The committed effective dose, H 50, for an adult man reached 16 nSv, which is lower than 0.001% of the background dose. The dose for the thyroid of an adult reached 0.33 μSv, which corresponds to circa 0.0007% of the dose to the population of Southern Poland after the Chernobyl nuclear plant accident.
Similar content being viewed by others
Introduction
On 11 March 2011, a 9.0 magnitude earthquake took place off the Pacific coast of Tōhoku, Japan. Subsequent 13-m-high tsunami waves damaged the cooling system (Tanaka 2012; Lipscy et al. 2013) at the Fukushima Daiichi Nuclear Power Plant. As a consequence, a radioactive emission occurred on the 12th of March containing mainly 131I, 133I, 134Cs, 137Cs, and other volatile species, which were transported in the atmosphere towards the north-western direction (Tanaka 2012). The accident was classified in the International Nuclear Event Scale as a level 7 accident. Previously, only the Chernobyl accident was rated at this level. During this accident, 1.8 × 1018 Bq of 131I were released into the environment (UNSCEAR 2000), whereas during the Fukushima accident 0.12 × 1018 Bq of 131I were released (USCEAR 2014).
The first traces from the Fukushima radioactive plume in Europe were detected in Reykjavik, Iceland, on the 20th of March (Bossew et al. 2012). On the morning of the 24th of March, the Laboratory of Radioactivity Analyses in Krakow, Poland, initiated an emergency mode for observations of radionuclides in the atmosphere; both aerosols and a gaseous fraction of 131I were considered (Mietelski et al. 2014).
131I is a beta-emitting radioisotope with a physical half-life of 8.021 days. The mean energy of electrons released during 131I nuclear decay is 606 keV. 131I is a nuclear fission product and as such it is commonly present in radioactive releases due to nuclear power plant failures. Furthermore, it is believed to contribute significantly to health hazards related to radioactive fallout. Being an iodine radioisotope, 131I is easily and preferentially concentrated in the human thyroid; note that the thyroid accumulates 30% of the total intake of iodine (Johansson et al. 2003) which is necessary for the proper functioning of the thyroid.
The International Commission on Radiological Protection (ICRP) developed a general methodology to calculate dose conversion factors and dose coefficients. This methodology was applied in the present study to estimate doses received by an average Polish citizen due to inhalation of 131I from the Fukushima fallout. More specifically, to quantify the incorporation of aerosol and gaseous fractions of radioiodine in the human organism, the age-dependent systemic biokinetic model proposed by Leggett (2010) was combined with the ICRP human respiratory tract model (HRTM) (ICRP 1994) and the ICRP gastrointestinal tract model (GITM) (ICRP 1979).
Materials and method
A general method of dose calculation is presented in this section. First, 131I concentration in the air was measured. Second, gaseous and aerosol 131I fractions deposited in various lung regions were estimated. Next, a complete biokinetic model for modelling the translocation of the deposited radionuclide within a human body following inhalation was constructed. Finally, ICRP recommendations were applied to evaluate equivalent and effective doses.
131I measurements
Iodine from the air was collected at the Institute of Nuclear Physics in Krakow, Poland, by a combined aerosol sampler MASS-500/gas sampler, operated at a flow rate of 250 m3 h−1 (Mietelski et al. 2014; Masson et al. 2011). The sorbent type for the aerosol fraction was a Petryanov filter FPP-15-1.5 (poly(vinyl chloride)) which has good aerosol-collecting properties: The aerosol collection efficiency of the Petryanov filter for aerosols with diameters 0.3 µm and for a linear velocity of the air passing through the filter of about 0.36 m s−1 reached 96.7% (Lipiński et al. 2013). For collection of the gas fraction, a sorbent of granular activated carbon impregnated with KI (IBJ-6, mesh size 2 mm, produced by Gryskand, Hajnówka, Poland) was used. The average efficiency of gaseous iodine adsorption reached (67 ± 11)% (Table 1). The use of activated carbon impregnated with KI allowed collection of both organic and inorganic iodine (Wilhelm 1982; Wangchang et al. 1993).
Between the 21st of March and 12th of April, the sorbents were changed every day. The filters and the granulated carbon from the gas cassettes were analysed by means of a low-background gamma spectrometer with HPGe detectors. Prior to measurements, the filters were compressed into pellets of 5 cm diameter and about 4 mm height. The carbon from each cassette was transferred into 0.5-L plastic Marinelli beakers and measured. Isotopes such as 131I, 132I, 129mTe, 132Te, 134Cs, 136Cs, and 137Cs were detected in the filters. The maximum activity for aerosols was equal to (5.73 ± 0.35), (0.461 ± 0.041) and (0.436 ± 0.038) mBq m−3 for 131I, 134Cs, and 137Cs, respectively, as observed on 29 March 2011. The detailed results are presented in Table 1 (Masson et al. 2011, 2013; Mietelski et al. 2014).
Deposition in the human respiratory tract
In the present study, the HRTM published in ICRP (1994) was used. In this approach, the human respiratory tract is divided into four anatomical regions, namely the extrathoracic region ET (consists of two parts: ET1 comprising interior nose and ET2 comprising posterior nasal passages, larynx, pharynx, and mouth), the bronchial region BB, the bronchiolar region bb, and the alveolar–interstitial region AI. The deposition of 131I aerosol fractions was calculated for five activity median aerodynamic diameters (AMADs) of the attached aerosols that were observed in Southern Poland after the Fukushima accident, i.e. 0.35, 0.36, 0.38, 0.47, and 0.53 μm (Masson et al. 2013). The deposition was calculated by a logarithmic interpolation of the deposition fraction values published by the ICRP in the HRTM using a six-term exponential function. Exemplary depositions for an adult male and a 0.35 μm aerosol AMAD are presented in Table 2.
In the gas fraction, iodine in elemental and organic form is absorbed in the respiratory tract in various proportions. However, when it is not possible to distinguish them (as was the case in the present study), the ICRP recommends adoption of the deposition rate for elemental iodine; accordingly, 100% deposition in the respiratory tract for elemental iodine was assumed in the present study with the respective distribution of 10% ET1, 40% ET2, and 50% BB (ICRP 1995). This simplification resulted in an overestimation of the calculated doses. In general, the fractional deposition in the respiratory tract regions was estimated for six age groups, both genders and four exercise levels, notably sitting, sleeping, light exercise, and heavy exercise. The typical time-budget distribution of different activities for 3-month infants was assumed as 71–29% for sleeping and light exercises, respectively, while the typical time budget for an adult male was assumed to be 33, 25, 41, and 1% for sleeping, sitting, light exercise, and heavy exercise, respectively. The average breathing rate for an adult man is 22.2 m3 day−1, while the breathing rates for the other age groups are 2.86 m3 day−1 for 3-month-old infants, 5.16 m3 day−1 for 1-year-olds, 8.72 m3 day−1 for 5-year-olds, 15.3 m3 day−1 for 10-year-olds, 20.1 m3 day−1 for 15-year-old males, 18 m3 day−1 for 15-year-old females, and 17.8 m3 day−1 for adult females. These breathing rates and other detailed ventilation parameters used in the model were taken from ICRP (2002) and are presented in Table 3.
Biokinetic models
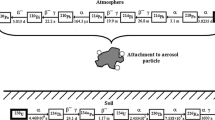
To describe the behaviour of the inhaled 131I in the human body and iodine accumulation in organs and tissues, the systemic biokinetic compartment model by Leggett (2010), the HRTM, and GITM were combined together into a one-compartment model. Such a solution has already been successfully applied for calculation of doses due to inhalation of indoor short-lived radon (both 222Rn and 220Rn) progenies for members of the public (Lie et al. 2008; Brudecki et al. 2014). The model is based on three subsystems for extrathyroidal inorganic iodine, thyroidal iodine, and extrathyroidal organic iodine. The model flow chart is presented in Fig. 1.
In the model, transfer of organic and hormonal iodine between compartments is characterized by transfer coefficients defined as the transferred fraction of the source compartment content per unit time. The factors used here are presented in Table 4. It is presupposed that inhaled iodine is quickly and entirely absorbed into the blood, where the iodine in red blood cells (RBCs) and plasma is assumed to be the same; therefore, the blood iodine is treated as a mixed pool. Another assumption is that iodine is excreted solely through urine and faeces (more than 90% of iodine removal is caused by renal clearance). Sweat secretion seems to be a negligible contribution to iodine loss. It is worth pointing out that only liver and kidney compartments were divided into two regions representing organic and inorganic iodide. The reasons for such specification are that kidneys are a greater extrathyroidal repository of organic and inorganic iodine, whereas liver stores much more hormonal iodine than any other extrathyroidal organ.
The model proposed by Leggett in 2010 is a biokinetic model for iodine for adults. Note that differences in the biokinetics of iodine between age groups are caused primarily by differences in iodine retention in the thyroid. The biological half-life of iodine for the thyroid is 11.2, 15, 23, 58, 67, and 90 days, for infants, 1-, 5-, 10-, and 15-year-old children and adults, respectively (ICRP 1993; Leggett 2010). In Leggett’s model, the time of iodine retention in the thyroid corresponds to the “Thyroid 2 to 2 Blood” parameter. Based on the values for biological half-life of iodine given above, this factor corresponds to 0.0619, 0.0462, 0.0301, 0.0119, 0.0103, and 0.0077 day−1 for infants, 1-, 5-, 10-, and 15-year-old children and adults, respectively.
The biokinetic behaviour of the inhaled iodine in the respiratory tract and other organs can be described by a system of first-order linear differential equations. The iodine activity in tissue and organs at any time after intake can be calculated numerically by solving these equations. The time-integrated iodine activity \(\tilde{A}\) in the thyroid and in other organs was calculated hereby integrating the activity in the organ up to the age of 70 years for children and over the 50-year commitment period following the intake for adults. The system of first-order differential equations was solved using the commercially available software SAAM II (Epsilon Group, VA, USA) (Barrett et al. 1998).
Equivalent dose and effective dose
The general methodology for dose calculation developed by the ICRP was also applied in the present study. Equivalent thyroid doses were calculated based on the time-integrated activity \(\tilde{A}\) and radiation-weighted S W factors. In general, the equivalent dose H(r T , T D ) in any target organ r T at age T integrated over the dose integration period T D is given by Eq. (1) (Bolch et al. 2009)
where A(r S , t) is the time-dependent activity in the source region r S at time t; S W(r T ← r S , t) is the radiation-weighted S factor, which depends on the radiation-weighting factor w R and energy E R of radiation R. S is calculated as given in Eq. (2)
For a given age group, S W(r T ← r S , t) is constant over the time interval T age D and \(\tilde{A}\) may be calculated as (Eq. 3)
Consistently, the equivalent dose in a specific age group is (Eq. 4)
Finally, the equivalent dose is calculated according to Eq. (5) by summing all the equivalent doses of each time interval as
Subsequently, the effective dose for a certain gender, E, may be calculated by Eq. (6) by summing equivalent doses, H T , for every target region and the tissue-weighting factor, w T
Following the new ICRP recommendations (ICRP 2007), the effective dose, E, for adults is calculated from the average of adult female (\(H_{T}^{\text{Female}}\)) and adult male (\(H_{T}^{\text{Male}}\)) equivalent doses (Eq. 7)
In the present investigation, the effective doses for adults and 15-year-olds are estimated based on the adequate female and male equivalent doses and ICRP 103 (2007) recommendations. For the other age groups, i.e. infants, 1-, 5-, and 10-year-olds, the effective doses are calculated without taking into consideration gender differences, according to ICRP (1991).
In practice, the S W value was calculated using the SEECAL program (Oak Ridge National Laboratory, Oak Ridge, TN, USA). The time-dependent activity, A(r S , t), was obtained by modelling for separate anatomical regions (Fig. 1) and the w T values used for calculations were taken from ICRP 60 (1991). Tissue-weighting factors, w T , were taken from ICRP 60 (1991) instead of ICRP 103 (2007), to allow validation of the model and comparison of the results with ICRP dose coefficients (ICRP 1995).
Validation of the method
To validate the methods applied, the estimated doses were compared with those obtained using ICRP dose coefficients (ICRP 1995) for the gaseous fraction of 131I. Results are presented in Table 5. The biggest differences are observed for infants (36%) and 1-year-old children (16%). In the other age groups, differences are around 10%. Such discrepancies occurred owing to the fact that Leggett’s model is more accurate than the current ICRP model proposed by Riggs (1952).
Results and discussion
In the present study, doses to South Poland residents due to inhalation of 131I were calculated, based on the 131I measured in air in South Poland after the nuclear power plant accident in Fukushima. The resulting effective doses and equivalent doses for the thyroid obtained for six age groups, namely 3-month infants, 1-, 5-, 10-, 15-year-old, and adults, as well as both genders for adults and 15-year-old teenagers, are shown in Tables 6 and 7.
With regard to the aerosol fraction, as expected the highest equivalent dose was achieved for the thyroid. The estimated doses for this organ were equal to 1.0 × 10−7, 1.9 × 10−7, 1.5 × 10−7, 1.0 × 10−7, 7.7 × 10−8, 6.9 × 10−8, 5.7 × 10−8, and 4.6 × 10−8 Sv for infants, 1-, 5-, 10-year-old children, 15-year-old males, 15-year-old females, male and female adults, respectively. The equivalent doses received by other body parts usually did not exceed hundreds of pSv. The largest effective dose (9.6 nSv) was calculated for a 1-year-old child, while for older ages, lower values were calculated (Fig. 2).
As far as the gaseous fraction of 131I is concerned, the thyroid was the organ with the highest equivalent doses, similarly to the case of the aerosol fraction. The thyroid equivalent doses were equal to 3.8 × 10−7, 5.5 × 10−7, 5.1 × 10−7, 4.5 × 10−7, 3.9 × 10−7, 3.4 × 10−7, 2.7 × 10−7, and 2.1 × 10−7 Sv for infants, 1-, 5-, 10-year-old children, 15-year-old males, 15-year-old females, male and female adults, respectively. Corresponding effective doses ranged from 28 nSv for a 1-year-old child to 11 nSv for an adult female (Fig. 3).
It should be noted that the doses calculated due to the gaseous fraction are overestimated. As already mentioned above, elemental and organic iodine in the gas fraction are absorbed in the respiratory tract in various proportions: 100% of elemental iodine is absorbed while only 70% of organic iodine is absorbed. For an adult male, the difference between the effective dose due to inhalation of elemental iodine and organic iodine is 25% (ICRP 1995). Because we were not able to distinguish between elemental and organic iodine in the gas fraction, it was assumed that all was elemental iodine and a 100% absorption was used, consistent with the recommendations of the ICRP. This led to a conservative dose assessment. Considering that organic iodine constitutes up to 86% of the total gaseous fraction (Noguchi and Murata 1988), doses estimated in the present study were probably overestimated by up to about 23%.
In general, the effective doses from iodine in the gaseous fraction are higher than those from iodine attached to aerosols: The gas-to-aerosol effective dose ratios are equal to 3.6, 2.9, 3.5, 4.4, 5.0, 4.8, and 4.7 for infant, 1-, 5-, 10-year-old children, 15-year-old boys and girls, adult men and women, respectively. Such differences in dose between the gaseous and aerosol fractions are caused by differences in deposition in the respiratory system. Accumulation of volatile (gaseous) iodine is between 70% and 100%, while the maximum deposition of particulate iodine (iodine attached to aerosols) observed in Southern Poland is on the 43% level for 0.53-μm-diameter particles for a 1-year-old child.
The sum of the thyroid doses induced by the iodine attached to aerosols or present in the gaseous fraction (and assuming 100% absorption) is equal to 0.48, 0.74, 0.66, 0.55, 0.46, 0.41, 0.33, and 0.26 μSv for infants, 1-, 5-, 10-year-old children, 15-year-old boys and girls, adult men and women, respectively. Compared with inhalation doses of 131I by Polish residents after the Chernobyl nuclear accident, when equivalent doses for the thyroid reached 178, 120, and 45 mSv for 5-year-old children, 10-year-old children, and adults, respectively (Pietrzak-Flis et al. 2003), the values due to the Fukushima accident are practically negligible. Similarly, the effective doses estimated here for Southern Poland are significantly lower than those estimated for the inhabitants of Fukushima and Japan: Depending on the residence place, thyroid doses for Japanese people were estimated as (0.1 ÷ 4.3), (0.1 ÷ 5.9), and (0.2 ÷ 7.5) mSv for adults, 10-year-old children, and 1-year-old children, respectively (UNSCEAR 2014). The doses found in the present study are consistent with those estimated by WHO (2012), who estimated for areas outside Japan effective doses of less than 0.01 mSv.
In the present paper, dose evaluation was performed for adults and 15-year-old teenagers for male and female separately, although ICRP recommends (ICRP 103) to calculate doses for adults as the average of the two genders (see Eq. 7). Differences found between these two approaches are presented in Table 8; the biggest discrepancy reached 13%.
Conclusions
In the present study, doses due to 131I originating from the Fukushima Daiichi Nuclear Power Plant accident inhaled by residents of Southern Poland were estimated. The doses were calculated for both 131I in aerosol and 131I in gaseous fractions. The dose values due to volatile iodine in the gaseous fraction were found to be higher than those estimated for particulate iodine (i.e. iodine attached to aerosols); the difference can be attributed to differences in the deposition of the discussed fractions in the human respiratory tract. The effective committed dose, H 50, for a male adult reached 16 nSv, which is less than 0.001% of the local background dose. The thyroid dose for an adult reached 0.33 μSv, which is less than 0.0007% of the dose that had been estimated for residents of Southern Poland, due to the Chernobyl accident. The doses to the inhabitants of Southern Poland did by no means represent any health hazard. Moreover, 131I air concentrations were comparable in most European countries (especially Central and Eastern parts of Europe). Therefore, the presented dose calculations are representative not only for Poles but also for most Europeans.
References
Barrett PHR, Bell BM, Cobelli C, Golde H, Schumitzky A, Vicini P, Foster DM (1998) SAAM II: simulation, analysis, and modelling software for tracer and pharmacokinetic studies. Metabolism 47:484–492
Bolch WE, Eckerman KF, Sgouros G, Thomas SR (2009) MIRD pamphlet no. 21: A Generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. J Nucl Med 50:477–484
Bossew P, Kirchner G, De Cort M, de Vries G, Nishev A, de Felice L (2012) Radioactivity from Fukushima Dai-ichi in air over Europe; part 1: spatio-temporal analysis. J Environ Radioact 114:22–34
Brudecki K, Li WB, Meisenberg O, Tschiersch J, Hoeschen C, Oeh U (2014) Age-dependent inhalation doses to members of the public from indoor short-lived radon progeny. Radiat Environ Biophys 53:535–549
ICRP (1979) International Commission of Radiological Protection. Limits for intakes of radionuclides by workers. Part 1. ICRP Publication 30. Annual ICRP 2(3–4). Pergamon, Oxford
ICRP (1991) International Commission on Radiological Protection. Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Annual ICRP 21(1–3) Pergamon, Oxford
ICRP (1993) International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides. Part 2 ingestion dose coefficients. ICRP Publication 67. Annual ICRP 23(3–4). Pergamon, Oxford
ICRP (1994) International Commission of Radiological Protection. The human respiratory tract model for radiological protection. ICRP Publication 66. Annual ICRP 24(1–3). Pergamon, Oxford
ICRP (1995) International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides: part 4. Inhalation dose coefficients. ICRP Publication 71. Annual ICRP 25(3–4). Pergamon, Oxford
ICRP (2002) International Commission of Radiological Protection. Guide for the practical application of the ICRP human respiratory tract model. ICRP Supporting Guidance 3. Pergamon, Oxford
ICRP (2007) International Commission on Radiological Protection. Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Annual ICRP 37(2–4) Pergamon, Oxford
Johansson L, Leide-Svegborn S, Mattsson S, Nosslin B (2003) Biokinetics of iodide in man: refinement of current ICRP dosimetry models. Cancer Biother Radiopharm 18(3):445–450
Leggett RW (2010) A physiological systems model for iodine for use in radiation protection. Radiat Res 174:496–516
Lie WB, Tschiersch J, Oeh U, Hoeschen C (2008) Lung dosimetry of inhaled thoron decay products. IN: 12th international congress of IRPA 2008, 19–24 October 2008, Buenos Aires, Argentina
Lipiński P, Isajenko K, Boratyński A, Fujak M, Piotrowska B (2013) The aspects of the air sampling. Regional workshop on regulatory control of radioactive discharges to the environment, 17–21 June 2013, Warsaw, Poland
Lipscy PY, Kushida KE, Incerti T (2013) The Fukushima disaster and Japan’s nuclear plant vulnerability in comparative perspective. Environ Sci Technol 47:6082–6088
Masson O, Baeza A, Bieringer J, Brudecki K, Bucci S, Cappai M, Carvalho FP, Connan O, Cosma C, Dalheimer A, Didier D, Depuydt G, De Geer LE, De Vismes A, Gini L, Groppi F, Gudnason K, Gurriaran R, Hainz D, Halldórsson O, Hammond D, Hanley O, Holeý K, Homoki Z, Ioannidou A, Isajenko K, Jankovic M, Katzlberger C, Kettunen M, Kierepko R, Kontro R, Kwakman PJM, Lecomte M, Leon Vintro L, Leppänen AP, Lind B, Lujaniene G, Mc Ginnity P, Mc Mahon C, Malá H, Manenti S, Manolopoulou M, Mattila A, Mauring A, Mietelski JW, Møller B, Nielsen SP, Nikolic J, Overwater RMW, Pálsson SE, Papastefanou C, Penev I, Pham MK, Povinec PP, Ramebäck H, Reis MC, Ringer W, Rodriguez A, Rulík P, Saey PRJ, Samsonov V, Schlosser C, Sgorbati G, Silobritiene BV, Söderström C, Sogni R, Solier L, Sonck M, Steinhauser G, Steinkopff T, Steinmann P, Stoulos S, Sýkora I, Todorovic D, Tooloutalaie N, Tositti L, Tschiersch J, Ugron A, Vagena E, Vargas A, Wershofen H, Zhukova O (2011) Tracking of airborne radionuclides from the damaged Fukushima Dai-ichi nuclear reactors by European networks. Environ Sci Technol 45:7670–7677
Masson O, Ringer W, Mala H, Rulik P, Dlugosz-Lisiecka M, Eleftheriadis K, Meisenberg O, Vismes-Ott A, Gensdarmes F (2013) Size distributions of airborne radionuclides from the Fukushima nuclear accident at several places in Europe. Environ Sci Technol 47:10995–11003
Mietelski JW, Kierepko R, Brudecki K, Janowski P, Kleszcz K, Tomankiewicz E (2014) Long-range transport of gaseous 131I and other radionuclides from Fukushima accident to Southern Poland. Atmos Environ 91:137–145
Noguchi H, Murata M (1988) Physicochemical speciation of airborne 131I in Japan from Chernobyl. J Environ Radioact 7:65–74
Pietrzak-Flis Z, Krajewski P, Radwan I, Muramatsu Y (2003) Retrospective evaluation of 131I deposition density and thyroid dose in Poland after the Chernobyl accident. Health Phys 84:698–708
Riggs DS (1952) Quantitative aspects of iodine metabolism in man. Pharmacol Rev 4:284–370
Tanaka S (2012) Accident at the Fukushima Dai-ichi nuclear power stations of TEPCO: outline & lessons learned. Proc Jpn Acad Ser B 8:471–484
UNSCEAR (2000) United Nations Scientific Committee on the effects of atomic radiation. Sources, effects and risks of ionizing radiation. UNSCEAR 2000 report to the general assembly, with scientific annexes, vol II
UNSCEAR (2014) United Nations Scientific Committee on the effects of atomic radiation. Sources, effects and risks of ionizing radiation. UNSCEAR 2013 report to the general assembly with scientific annexes
Wangchang L, Yuying H, Yianwei W, Ming J, Liangtian G (1993) Research on removal of radioiodine by charcoal. In: L/ILW management and final disposal proceedings. China, p 294
WHO (2012) World Health Organization. Preliminary dose estimation from the nuclear accident after the 2011 Great East Japan earthquake and tsunami
Wilhelm JG (1982) Iodine filters in nuclear installations. Commission of the European Communities. Luxemburg
Acknowledgements
This study partly was funded by the National Science Centre, Poland (Grant Number 2014/15/B/NZ7/00925).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kamil Brudecki, Katarzyna Szufa, and Jerzy Wojciech Mietelski declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brudecki, K., Szufa, K. & Mietelski, J.W. 131I age-dependent inhalation dose in Southern Poland from Fukushima accident. Radiat Environ Biophys 56, 9–17 (2017). https://doi.org/10.1007/s00411-016-0672-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-016-0672-3