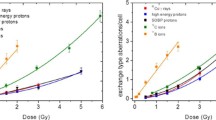
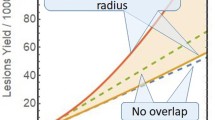
Abstract
When fractionation schemes for hypofractionation and stereotactic body radiotherapy are considered, a reliable cell survival model at high dose is needed for calculating doses of similar biological effectiveness. In this work, a simple model for cell survival which is valid also at high dose is developed from Poisson statistics. It is assumed that a cell is killed by an event that is defined by two double-strand breaks on the same or different chromosomes. Two different mechanisms can produce events. A one-track event is always represented by two simultaneous double-strand breaks. A two-track event results in one double-strand break. Therefore, at least two two-track events on the same or different chromosomes are necessary to produce an event. It is assumed that two double-strand breaks can be repaired with a certain repair probability. Both the one-track events and the two-track events are statistically independent. From the stochastic nature of cell killing which is described by the Poisson distribution, the cell survival probability was derived. The model was fitted to experimental data. It was shown that a solution based on Poisson statistics exists for cell survival. It exhibits exponential cell survival at high dose and a finite gradient of cell survival at vanishing dose, which is in agreement with experimental cell studies. The model fits the experimental data as well as the LQ model and is based on two free parameters. It was shown that cell survival can be described with a simple analytical formula on the basis of Poisson statistics. This solution represents in the limit of large dose the typical exponential behavior and predicts cell survival as well as the LQ model.








Similar content being viewed by others
References
Algan O, Stobbe CC, Helt AM, Hanks GE, Chapman JD (1996) Radiation inactivation of human prostate cancer cells: the role of apoptosis. Radiat Res 146(3):267–275
Alper T (1979) Cellular radiobiology. Cambridge University Press, Cambridge
Astrahan M (2008) Some implications of linear-quadratic-linear radiation dose-response with regard to hypofractionation. Med Phys 35(9):4161–4172
Atwood K, Norman A (1949) On the interpretation of multi-hit survival curves. Proc Natl Acad Sci USA 35:696–709
Besserer J, Schneider U (2014) A track-event theory of cell survival. Z Med Phys. doi:10.1016/j.zemedi.2014.10.001 [Epub ahead of print]
Brenner DJ (2008) The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 18(4):234–239
Carlone M, Wilkins D, Raaphorst G (2005) The modified linear quadratic model of Guerrero and Li can be derived from mechanistic basis and exhibits linear-quadratic-linear behavior. Phys Med Biol 50:L9–L15
Chapman JD, Blakely EA, Smith KC, Urtasun RC, Lyman JT, Tobias CA (1978) Radiation biophysical studies with mammalian cells and a modulated carbon ion beam. Radiat Res 74(1):101–111
Dale RG (1985) The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol 58(690):515–528
DeWeese TL, Shipman JM, Dillehay LE, Nelson WG (1998) Sensitivity of human prostatic carcinoma cell lines to low dose rate radiation exposure. J Urol 159(2):591–598
Douglas BG, Fowler JF (1976) The effect of multiple small doses of X rays on skin reactions in the mouse and a basic interpretation. Radiat Res 66(2):401–426
Ekstrand KE (2010) The Hug–Kellerer equation as the universal cell survival curve. Phys Med Biol 55(10):N267–N273
Elkind M, Sutton H (1959) X-ray damage and recovery in mammalian cells in culture. Nature 184:1293–1295
Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62(740):679–694
Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H, Yatagai F, Kanai T, Ando K (2000) Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat Res 154(5):485–496. Erratum in: Radiat Res. 2012 Jan;177(1):129–131
Garcia LM, Leblanc J, Wilkins D, Raaphorst GP (2006) Fitting the linear-quadratic model to detailed data sets for different dose ranges. Phys Med Biol 51(11):2813–2823
Hall EJ, Marchese MJ, Astor MB, Morse T (1986) Response of cells of human origin, normal and malignant, to acute and low dose rate irradiation. Int J Radiat Oncol Biol Phys 12(4):655–659
Hug O, Kellerer AM (1963) Zur Interpretation der Dosiswirkungsbeziehungen in der Strahlenbiologie. Biophysik 1:20–32
Ito A, Nakano H, Kusano Y, Hirayama R, Furusawa Y, Murayama C, Mori T, Katsumura Y, Shinohara K (2006) Contribution of indirect action to radiation-induced mammalian cell inactivation: dependence on photon energy and heavy-ion LET. Radiat Res 165(6):703–712
Kamlah F, Hänze J, Arenz A, Seay U, Hasan D, Juricko J, Bischoff B, Gottschald OR, Fournier C, Taucher-Scholz G, Scholz M, Seeger W, Engenhart-Cabillic R, Rose F (2011) Comparison of the effects of carbon ion and photon irradiation on the angiogenic response in human lung adenocarcinoma cells. Int J Radiat Oncol Biol Phys 80(5):1541–1549
Kellerer AM, Rossi HH (1972) The theory of dual radiation action. Curr Top Radiat Res 8:85–158
Lea DE, Catcheside D (1942) The mechanism of the induction by radiation chromosome aberrations in tradescantia. J Genet 44:216
Leith JT, Quaranto L, Padfield G, Michelson S, Hercbergs A (1993) Radiobiological studies of PC-3 and DU-145 human prostate cancer cells: X-ray sensitivity in vitro and hypoxic fractions of xenografted tumors in vivo. Int J Radiat Oncol Biol Phys 25(2):283–287
Miyakawa A, Shibamoto Y, Otsuka S, Iwata H (2014) Applicability of the linear-quadratic model to single and fractionated radiotherapy schedules: an experimental study. J Radiat Res 55(3):451–454
Park C, Papiez L, Zhang S, Story M, Timmerman RD (2008) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 70(3):847–852
Persson LM, Edgren MR, Stenerlöw B, Lind BK, Hedlöf I, Jernberg AR, Meijer AE, Brahme A (2002) Relative biological effectiveness of boron ions on human melanoma cells. Int J Radiat Biol 78(8):743–748
Puck T, Markus P (1956) Action of X-rays on mammalian cells. J Exp Med 103:653–666
Ruiz de Almodóvar JM, Bush C, Peacock JH, Steel GG, Whitaker SJ, McMillan TJ (1994) Dose-rate effect for DNA damage induced by ionizing radiation in human tumor cells. Radiat Res 138(1 Suppl):S93–S96
Sachs RK, Hahnfeld P, Brenner DJ (1997) The link between low-LET dose-response relations and the underlying kinetics of damage production/repair/misrepair. Int J Radiat Biol 72(4):351–374
Schalch T, Duda S, Sargent DF, Richmond TJ (2005) X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature 436(7047):138–141
Steel GG, Deacon JM, Duchesne GM, Horwich A, Kelland LR, Peacock JH (1987) The dose-rate effect in human tumour cells. Radiother Oncol 9(4):299–310
Stenerlöw B, Pettersson OA, Essand M, Blomquist E, Carlsson J (1995) Irregular variations in radiation sensitivity when the linear energy transfer is increased. Radiother Oncol 36(2):133–142
Sullivan FJ, Carmichael J, Glatstein E, Mitchell JB (1996) Radiation biology of lung cancer. J Cell Biochem 24:152–159
Suzuki M, Kase Y, Kanai T, Yatagai F, Watanabe M (1997) LET dependence of cell death and chromatin-break induction in normal human cells irradiated by neon-ion beams. Int J Radiat Biol 72:497–503
Tonkin KS, Kelland LR, Steel GG (1989) A comparison of the in vivo and in vitro radiation response of three human cervix carcinomas. Radiother Oncol 16(1):55–63. Erratum in: Radiother Oncol 1989 Oct;16(2):157
Tsuboi K, Tsuchida Y, Nose T, Ando K (1998) Cytotoxic effect of accelerated carbon beams on glioblastoma cell lines with p53 mutation: clonogenic survival and cell-cycle analysis. Int J Radiat Biol 74:71–79
Tsuchida Y, Tsuboi K, Ohyama H, Ohno T, Nose T, Ando K (1998) Cell death induced by high-linear-energy transfer carbon beams in human glioblastoma cell lines. Brain Tumor Pathol 15(2):71–76
Woodcock CL (2005) A milestone in the odyssey of higher-order chromatin structure. Nat Struct Mol Biol 12(8):639–640
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Besserer, J., Schneider, U. Track-event theory of cell survival with second-order repair. Radiat Environ Biophys 54, 167–174 (2015). https://doi.org/10.1007/s00411-015-0584-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-015-0584-7