Abstract
Background
Response–dose ratio (RDR) and cumulative provocative dosage (PD) are useful indices reflecting airway responsiveness in asthma.
Objectives
To compare the diagnostic value of RDR and PD, by conducting leukotriene D4 (LTD4-BPT) and methacholine bronchial provocation test (MCh-BPT), in different asthma control levels.
Methods
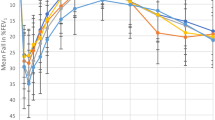
Healthy subjects and asthmatic patients underwent LTD4-BPT and MCh-BPT, at 2–14-day interval. This entailed assessment of the distribution characteristics, correlation, and diagnostic value of PD inducing 20 % fall in forced expiratory volume in one second (PD20FEV1) and the RDR, defined as FEV1 fall (%) at the final step divided by the corresponding provocative dosage.
Results
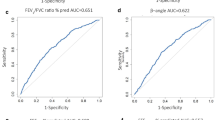
Twenty uncontrolled, 22 partly controlled, 20 controlled asthmatics, and 21 healthy subjects were enrolled. Log10RDR was positively correlated with log10PD20FEV1 in both BPTs (all P < 0.05). Poorer asthma control was associated with significantly lower PD20FEV1 and higher RDR (both P < 0.05). The differences in PD20FEV1 and RDR between partly controlled and controlled asthma were unremarkable (both P > 0.05). Compared with log10PD20FEV1, the log10RDR yielded similar diagnostic values in both BPTs. A lower percentile of RDR (≤25th percentile) was associated with higher baseline FEV1 (P < 0.05) and an increased proportion of well-controlled asthmatic patients. The combination of RDR and PD20FEV1 led to an increased diagnostic value compared with either parameter alone.
Conclusions
RDR is a surrogate of PD20FEV1 for BPTs in asthma. This finding was not modified by different asthma control levels or the types of bronchoprovocants.


Similar content being viewed by others
Abbreviations
- RDR:
-
Response–dose ratio
- FVC:
-
Forced vital capacity
- FEV1 :
-
Forced expiratory volume in one second
- PEF:
-
Peak expiratory flow
- LT:
-
Leukotriene
- LTD4-BPT:
-
Leukotriene D4 bronchial provocation test
- MCh-BPT:
-
Methacholine bronchial provocation test
- PD20FEV1-LTD4 :
-
Cumulative dose of leukotriene D4 causing a 20 % fall in FEV1
- PD20FEV1-MCh:
-
Cumulative dose of methacholine causing a 20 % fall in FEV1
- ROCC:
-
Receiver operation characteristic curve
- UC:
-
Uncontrolled
- PC:
-
Partly controlled
- CC:
-
Controlled
- μmol:
-
Micromole
- nmol:
-
Nanomoles
- UC-PD:
-
Provocative dosage in uncontrolled asthma group
- UC-RDR:
-
Response–dose ratio in uncontrolled asthma group
- PC-PD:
-
Provocative dosage in partly controlled asthma group
- PC-RDR:
-
Response–dose ratio in partly controlled asthma group
- CC-PD:
-
Provocative dosage in controlled asthma group
- CC-RDR:
-
Response–dose ratio in controlled asthma group
- HS-PD:
-
Provocative dosage in healthy subjects
- HS-RDR:
-
Response–dose ratio in healthy subjects
- Log10RDRMCh :
-
Logarithm of the RDR in MCh-BPT
- Log10RDRLTD4 :
-
Logarithm of the RDR in LTD4-BPT
References
O’Byrne PM, Inman MD (2003) Airway hyperresponsiveness. Chest 123:411S–416S
Toelle BG, Peat JK, Salome CM, Crane J, McMillan D, Dermand J et al (1994) Comparison of two epidemiological protocols for measuring airway responsiveness and allergic sensitivity in adults. Eur Respir J 7:1798–1804
Meer GD, Marks GB, Jongste JD, Brunekreef B (2005) Airway responsiveness to hypertonic saline: response-dose ratio or PD15? Eur Respir J 25:153–158
Juusela M, Pallasaho P, Sarna S, Piirilä P, Lundbäck B, Sovijärvi A (2013) Bronchial hyperresponsiveness in an adult population in Helsinki: decreased FEV1, the main determinant. Clin Respir J 7(1):34–44
Prieto L, Ferrer A, Domenech J, Perez-Frances C (2006) Effect of challenge method on sensitivity, reactivity, and maximal response to methacholine. Ann Allergy Asthma Immunol 97:175–181
O’Byrne PM (1997) Leukotrienes in the pathogenesis of asthma. Chest 111:27S–34S
Guan W, Zheng J, Gao Y, Jiang C, Xie Y, An J et al (2013) Leukotriene D4 and methacholine bronchial provocation test for identifying leukotriene-responsiveness subtypes. J Allergy Clin Immunol 131:332–338
O’Byrne PM, Bateman ED, Bousquet JD, Clark T, Ohta K, Paggiaro P, et al Global Initiative for Asthma (GINA) (2014) Global strategy for asthma management and prevention. Updated 2006. Available at http://www.ginasthma.org/Guidelines/guidelines-resources.html. Assessed 15 Feb 2014
Guan W, Zheng J, Gao Y, Jiang CY, An J, Yu X et al (2012) Leukotriene D4 bronchial provocation test: methodology and diagnostic value. Curr Med Res Opin 28:797–803
Laszio G (2005) ATS/ERS task force: standardization of spirometry. Eur Respir J 26:319–338
Zheng JP, Zhong NS (2002) Normative values of pulmonary function testing in Chinese adults. Chin Med J 115:50–54
Guan WJ, Zheng JP, Gao Y, Jiang CY, Shi X, Xie YQ et al (2013) Impulse oscillometry for leukotriene D4 inhalation challenge in asthma. Respir Care 12:2120–2126
Guan WJ, Zheng JP, Gao Y, Jiang CY, Xie YQ, Shi X et al (2013) Responsiveness to leukotriene D4 and methacholine for predicting efficacy of montelukast in asthma. J Thorac Dis 5:298–301
Guan WJ, Shi X, Zheng JP, Gao Y, Jiang CY, Xie YQ et al (2014) Leukotriene D4 inhalation challenge for predicting short-term efficacy of montelukast in asthma. Clin Respir J. doi:10.1111/crj.12117
Acknowledgments
The authors would like to thank Drs. Ke-fang Lai, Ru-chong Chen, and Wei Luo (State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, First Affiliated Hospital of Guangzhou Medical University) for their useful comments. This work is supported by the Provincial Nature Science Foundation of Guangdong 915280000100019 (Dr. J. Zheng), Municipal science and technology plan of Guangzhou 2009J1-C321-1 (Dr. Y. Gao), Guangzhou leading project for medicine and health 2009-YB-143 (Dr. Y. Gao) and the Scientific Project of Guangzhou Medical College 2008A02 (Dr. Y. Gao), Changjiang Scholars and Innovative Research Team in University ITR0961 (Dr. J. Zheng), and the National Key Technology R&D Program of the 12th National Five-year Development Plan (2012BAI05B01 and 2013BAI09B09) (Dr. J. Zheng).
Conflict of interest
Dr. Zheng and Gao received the Provincial Nature Science Foundation of Guangdong 915280000100019 (Dr. J. Zheng), Municipal science and technology plan of Guangzhou 2009J1-C321-1 (Dr. Y. Gao), Guangzhou leading project for medicine and health 2009-YB-143 (Dr. Y. Gao) and the Scientific Project of Guangzhou Medical College 2008A02 (Dr. Y. Gao), Changjiang Scholars and Innovative Research Team in University ITR0961 (Dr. J. Zheng), and the National Key Technology R&D Program of the 12th National Five-year Development Plan (2012BAI05B01 and 2013BAI09B09) (Dr. J Zheng). None of these funding had any influence on data collection or interpretation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guan, Wj., Zheng, Jp., Shi, X. et al. Response–Dose Ratio is a Surrogate of Cumulative Provocative Dosage for Bronchial Provocation Tests in Asthma. Lung 192, 701–709 (2014). https://doi.org/10.1007/s00408-014-9612-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-014-9612-7