Abstract
Background
Hedgehog signaling is known to be involved in both lung organogenesis and lung carcinogenesis. The aim of this study was to examine potential downstream targets of the hedgehog signaling pathway in non-small-cell lung cancer.
Methods
Protein expression of Bmi1, FoxF1, Nanog, and γ-catenin was examined by immunohistochemistry in 80 non-small-cell lung cancer samples. Correlations with the previously immunohistochemically recovered results for sonic hedgehog, Ptch1, Smo, Gli1, and Gli2 in the same cohort of tumors as well as the clinicopathological characteristics of the tumors were also evaluated.
Results
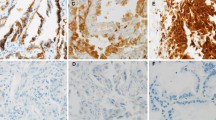
Bmi1 was expressed in 78/80 (97.5 %) cases of non-small-cell lung cancer and correlated with male gender and expression of Gli1. Positive expression of FoxF1 was found in 62/80 (77.5 %) cases. Expression of FoxF1 correlated with lymph node metastases, Bmi1, and hedgehog pathway activation. Overexpression of Nanog was also noted in 74/80 (92.5 %) tumors and correlated with Bmi1. Cytoplasmic accumulation of γ-catenin was observed in 85 % (68/80) of the tumors and correlated with the expression of Bmi1, FoxF1, and Nanog.
Conclusion
Several developmental pathways seem to be implicated in non-small-cell lung cancer. It is also suggested that Bmi1 and FoxF1 may cooperate with hedgehog signaling in non-small-cell lung carcinogenesis.


Similar content being viewed by others
References
Daniel VC, Peacock CD, Watkins DN (2006) Developmental signalling pathways in lung cancer. Respirology 11(3):234–240
van Tuyl M, Post M (2000) From fruitflies to mammals: mechanisms of signalling via the Sonic hedgehog pathway in lung development. Respir Res 1(1):30–35
Velcheti V, Govindan R (2007) Hedgehog signaling pathway and lung cancer. J Thorac Oncol 2(1):7–10
Ruiz i Altaba A, Stecca B, Sánchez P, Ruiz i Altaba A, Stecca B, Sánchez P (2004) Hedgehog–Gli signaling in brain tumors: stem cells and paradevelopmental programs in cancer. Cancer Lett 204(2):145–157
Kalderon D (2000) Transducing the hedgehog signal. Cell 103(3):371–374
Mullor JL, Sánchez P, Ruiz i Altaba A (2002) Pathways and consequences: hedgehog signaling in human disease. Trends Cell Biol 12(12):562–569
Gialmanidis IP, Bravou V, Amanetopoulou SG et al (2009) Overexpression of hedgehog pathway molecules and FOXM1 in non-small cell lung carcinomas. Lung Cancer. 66(1):64–74
Michael LE, Westerman BA, Ermilov AN et al (2008) Bmi1 is required for Hedgehog pathway-driven medulloblastoma expansion. Neoplasia 10(12):1343–1349
Zbinden M, Duquet A, Lorente-Trigos A et al (2010) NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO J 29(15):2659–2674
Yoon JW, Kita Y, Frank DJ et al (2002) Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem 277(7):5548–5555
Jacobs JJ, Kieboom K, Marino S et al (1999) The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397(6715):164–168
Park IK, Qian D, Kiel M et al (2003) Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423(6937):302–305
Molofsky AV, Pardal R, Iwashita T et al (2003) Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425(6961):962–967
Molofsky AV, He S, Bydon M et al (2005) Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 19(12):1432–1437
Prince ME, Sivanandan R, Kaczorowski A et al (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A 104(3):973–978
Liu JH, Song LB, Zhang X et al (2008) Bmi-1 expression predicts prognosis for patients with gastric carcinoma. J Surg Oncol 97(3):267–272
Vonlanthen S, Heighway J, Altermatt HJ et al (2001) The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer 84(10):1372–1376
Kaufmann E, Knöchel W (1996) Five years on the wings of fork head. Mech Dev 57(1):3–20
Stankiewicz P, Sen P, Bhatt SS et al (2009) Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet 84(6):780–791
Yamaguchi S, Kimura H, Tada M et al (2005) Nanog expression in mouse germ cell development. Gene Expr Patterns 5(5):639–646
Chiou SH, Wang ML, Chou YT et al (2010) Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res 70(24):10433–10444
Barker N, Clevers H (2000) Catenins. Wnt signaling and cancer. Bioessays 22(11):961–965
Wendling DS, Lück C, von Schweinitz D et al (2008) Characteristic overexpression of the forkhead box transcription factor Foxf1 in Patched-associated tumors. Int J Mol Med 22(6):787–792
Dlugosz AA, Talpaz M (2009) Following the hedgehog to new cancer therapies. N Engl J Med 361(12):1202–1205
Xu F, Yang R, Wu L et al (2012) Overexpression of BMI1 confers clonal cells resistance to apoptosis and contributes to adverse prognosis in myelodysplastic syndrome. Cancer Lett 317(1):33–40
Lo PK, Lee JS, Liang X et al (2010) Epigenetic inactivation of the potential tumor suppressor gene FOXF1 in breast cancer. Cancer Res 70(14):6047–6058
Saito RA, Micke P, Paulsson J et al (2010) Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res 70(7):2644–2654
Maeda Y, Davé V, Whitsett JA (2007) Transcriptional control of lung morphogenesis. Physiol Rev 87(1):219–244
Shakhova O, Leung C, Marino S (2005) Bmi1 in development and tumorigenesis of the central nervous system. J Mol Med 83(8):596–600
Grinstein E, Mahotka C (2009) Stem cell divisions controlled by the proto-oncogene BMI-1. J Stem Cells 4(3):141–146
Kalinichenko VV, Gusarova GA, Kim IM et al (2004) Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol 286(3):L521–L530
Mahlapuu M, Enerbäck S, Carlsson P (2001) Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 128(12):2397–2406
Teglund S (1805) Toftgård R (2010) Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2:181–208
Liu S, Dontu G, Mantle ID et al (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66(12):6063–6071
Nirasawa S, Kobayashi D, Tsuji N et al (2009) Diagnostic relevance of overexpressed Nanog gene in early lung cancers. Oncol Rep 22(3):587–591
Yuan P, Kadara H, Behrens C et al (2010) Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS ONE 5(2):e9112
Leung EL, Fiscus RR, Tung JW et al (2010) Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS ONE 5(11):e14062
Forte A, Schettino MT, Finicelli M et al (2009) Expression pattern of stemness-related genes in human endometrial and endometriotic tissues. Mol Med 15(11–12):392–401
Melone MA, Giuliano M, Squillaro T et al (2009) Genes involved in regulation of stem cell properties: studies on their expression in a small cohort of neuroblastoma patients. Cancer Biol Ther 8(13):1300–1306
Mathieu J, Zhang Z, Zhou W et al (2011) HIF induces human embryonic stem cell markers in cancer cells. Cancer Res 71(13):4640–4652
Aktary Z, Pasdar M (2012) Plakoglobin: role in tumorigenesis and metastasis. Int J Cell Biol 2012:189521
Nagel JM, Kriegl L, Horst D et al (2010) γ-Catenin is an independent prognostic marker in early stage colorectal cancer. Int J Colorectal Dis 25(11):1301–1309
Shahi MH, Afzal M, Sinha S et al (2010) Regulation of sonic hedgehog-GLI1 downstream target genes PTCH1, Cyclin D2, Plakoglobin, PAX6 and NKX2.2 and their epigenetic status in medulloblastoma and astrocytoma. BMC Cancer 10:614
Kolligs FT, Kolligs B, Hajra KM et al (2000) γ-Catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of beta-catenin. Genes Dev 14(11):1319–1331
Chikaishi Y, Uramoto H, Tanaka F (2011) The EMT status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res 31(12):4451–4456
Yamashita T, Uramoto H, Onitsuka T et al (2010) Association between lymphangiogenesis-/micrometastasis- and adhesion-related molecules in resected stage I NSCLC. Lung Cancer 70(3):320–328
Winn RA, Bremnes RM, Bemis L et al (2002) γ-Catenin expression is reduced or absent in a subset of human lung cancers and re-expression inhibits transformed cell growth. Oncogene 21(49):7497–7506
Shahi MH, Schiapparelli P, Afzal M et al (2011) Expression and epigenetic modulation of sonic hedgehog-GLI1 pathway genes in neuroblastoma cell lines and tumors. Tumour Biol 32(1):113–127
Conflict of interest
The authors have conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gialmanidis, I.P., Bravou, V., Petrou, I. et al. Expression of Bmi1, FoxF1, Nanog, and γ-Catenin in Relation to Hedgehog Signaling Pathway in Human Non-small-Cell Lung Cancer. Lung 191, 511–521 (2013). https://doi.org/10.1007/s00408-013-9490-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9490-4