Abstract
Background
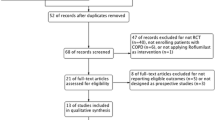
This is a meta-analysis of the safety and efficacy of indacaterol in chronic obstructive pulmonary disease (COPD) with treatment duration of ≥12 weeks.
Methods
Randomized controlled trials (RCTs) reported in English (to September 30, 2012) were identified from PubMed, the Cochrane Library, Embase, websites, reference lists, and manual searches. Two reviewers independently assessed the quality of the trials and extracted information.
Results
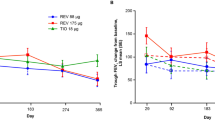
Five RCTs were eligible. Five involved indacaterol, two salmeterol, one formoterol, and one tiotropium. Four studies had placebos. Using trough forced expiratory volume in 1 s as a measure of therapeutic effect, indacaterol was superior to the other β2-agonists, tiotropium, and placebo at weeks 12, 26, and 52. Indacaterol had a greater effect on the transition dyspnoea index compared with placebo, formoterol, and salmeterol, but not open-label tiotropium. In reducing the as-needed use of salbutamol, indacaterol were superior to placebo, tiotropium, and formoterol, but not salmeterol (5, 95 % confidence interval (CI), –2.15, 12.15). Indacaterol improved St George’s Respiratory Questionnaire scores more than placebo and open-label tiotropium, but not formoterol. Indacaterol seemed to cause more adverse events than placebo only at a dose of 600 μg daily and a duration of 52 weeks (risk ratio 1.15; 95 % CI, 1.04, 1.26). The total and serious adverse events and adverse events leading to discontinuation were comparable with open-label tiotropium and the β2-agonists.
Conclusions
Indacaterol is effective and well-tolerated as a bronchodilator for the maintenance of moderate to severe COPD.













Similar content being viewed by others
References
World Health Organisation (WHO) World Health Statistics. Available at: http://www.who.int/whosis/whostat/EN WHS08 Full.pdf
Global initiative for chronic obstructive lung disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2011. Available at: www.goldcopd.com
Food and Drug Administration (FDA) FDA approves Arcapta Neohaler to treat chronic obstructive pulmonary disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm261649.htm
Maele B, Fabbri LM, Martin C, Horton R, Dolker M, Overend T (2010) Cardiovascular safety of QVA149, a combination of indacaterol and NVA237, in COPD patients. COPD 7:418–427. doi:10.3109/15412555.2010.528812
Vogelmeier C, Ramos-Barbon D, Jack D, Piggott S, Owen R, Higgins M et al (2010) Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium. Respir Res 11:135–142. http://respiratory-research.com/content/11/1/135
LaForce C, Aumann J, Parreño L, Iqbal A, Young D, Owen R et al (2011) Sustained 24-hour efficacy of once daily indacaterol (300 mg) in patients with chronic obstructive pulmonary disease: a randomized, crossover study. Pulm Pharmacol Ther 24:162–168
O’Donnell DE, Casaburi B, Vincken W, Puente-Maestu L, Swales J, Lawrence D et al (2011) Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med 105:1030–1036. doi:10.1016/j.rmed.2011.03.014
Magnussen H, Verkindre C, Jack D, Jadayel D, Henley M, Woessner R et al (2010) Indacaterol once a day is equally effective dosed in the evening or morning in COPD. Respir Med 104:1869–1876. doi:10.1016/j.rmed.2010.08.010
van Noord JA, Buhl R, LaForce C, Martin C, Jones F, Dolker M et al (2010) QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax 65:1086–1091. doi:10.1136/thx.2010.139113
Beier J, Chanez P, Martinot J-B, Schreurs AJ, Tkacova R, Bao W et al (2007) Safety, tolerability and efficacy of indacaterol, a novel once a day b2-agonist, in patients with COPD: a 28-day randomised, placebo controlled clinical trial. Pulm Pharmacol Ther 20:740–749. doi:10.1016/j.pupt.2006.09.001
Beeh K-M, Wagner F, Khindri S, Drollmann AF (2011) Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD 8:340–345. doi:10.3109/15412555.2011.594464
Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M et al (2010) Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther 23:165–171. doi:10.1016/j.pupt.2010.01.003
Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, Chuchalin A et al (2008) A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med 102:1033–1044. doi:10.1016/j.rmed.2008.02.001
Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C et al (2011) Indacaterol once a day provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med 105:719–726. doi:10.1016/j.rmed.2011.02.008
Feldman G, Siler T, Prasad N, Jack D, Piggott S, Owen R et al (2010) Efficacy and safety of indacaterol 150 microg once a day in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med 10:11–19. http://dx.doi.org/10.1186/1471-2466-10-11
Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorganciog ˘lu A et al (2010) Once a day bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 182(2):155–162. http://dx.doi.org/10.1164/rccm.200910-1500OC
Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D et al (2010) Efficacy of a new once a day long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 65(6):473–479. http://dx.doi.org/10.1136/thx.2009.125435
Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C et al (2011) Once a day indacaterol vs. twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 37:273–279 http://dx.doi.org/10.1183/09031936.00045810
Rossi A, Centanni S, Cerveri I, Gulotta C, Foresi A, Cazzola M et al (2011) Acute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropium. Respir Med 106(1):84–90. doi:10.1016/j.rmed.2011.09.006
Balint B, Watz H, Amos C, Owen R, Higgins M, Kramer B et al (2010) Onset of action of indacaterol in patients with COPD: Comparison with salbutamol and salmeterol–fluticasone. Int J Chron Obstruct Pulm Dis 5:311–318
Beier J, Beeh K-M, Brookman L, Peachey G, Hmissi A, Pascoe S (2009) Bronchodilator effects of indacaterol and formoterol in patients with COPD. Pulm Pharmacol Ther 22:492–496. doi:10.1016/j.pupt.2009.05.001
Kato M, Makita H, Uemura K, Fukuchi Y, Hosoe M, Emery C et al (2010) Bronchodilator efficacy of single doses of indacaterol in Japanese patients with COPD: a randomised, double-blind, placebo-controlled trial. Allergol Int 59:285–293. doi:10.2332/allergolint.10-OA-0173
Bauwens O, Ninane V, Van de Maele B, Firth R, Dong F, Owen R et al (2009) 24-hour bronchodilator efficacy of single doses of indacaterol in subjects with COPD: comparison with placebo and formoterol. Curr Med Res Opin 25(2):463–470. doi:10.1185/03007990802675096
Mahler DA, Witek TJ Jr (2005) The MCID of the transition dyspnea index is a total score of one unit. COPD 2:99–103
Witek TJ Jr, Mahler DA (2003) Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 21:267–272
Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T (2010) Development and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J 36(1):96–104. http://dx.doi.org/10.1183/09031936.00123709
Jones PW, Quirk FH, Baveystock CM, Littlejohns P (1992) A self-complete measure for chronic airflow limitation: the St George’s respiratory questionnaire. Am Rev Respir Dis 55(6):1321–1327
Jones PW (2005) St George’s respiratory questionnaire: MCID. COPD 2:75–83
Jones PW, Mahler DA, Gale R, Owen R, Kramer B (2011) Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respir Med 105(6):892–899
Jones PW, Barnes N, Vogelmeier C, Lawrence D, Kramer B (2011) Efficacy of indacaterol in the treatment of patients with COPD. Prim Care Respir J 20(4):380–388
Acknowledgments
The authors thank Professor WU Tai-xiang (Chinese Cochrane Center) for his kind assistance.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, FM., Liang, ZA., Zheng, QL. et al. Safety and Efficacy of 12-Week or Longer Indacaterol Treatment in Moderate-to-Severe COPD Patients: A Systematic Review. Lung 191, 135–146 (2013). https://doi.org/10.1007/s00408-012-9444-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-012-9444-2