Abstract
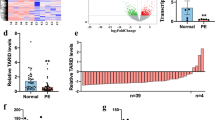
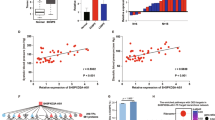
Preeclampsia, as one of the most serious pregnancy-specific diseases, manifested by high blood pressure and companied by proteinuria in pregnancy women after 20 gestational weeks. Although the underlying mechanism has been studied for decades, no unambiguous interpretation of this phenomenon was well recognized. Recent researches focused on long non-coding RNAs (lncRNAs) as key regulators of cancer cell proliferation, invasion, migration and angiogenesis. Tumor development and placenta implantation share several common biological behaviors. The expression of lncRNA MALAT1 was downregulated in the placenta of patients with severe preeclampsia. MALAT1 smart silencer significantly inhibited HTR-8/SVneo trophoblast cell proliferation, invasion, migration and tube formation in vitro. Moreover, MALAT1 inhibited the expression of angiogenic factors in umbilical vein endothelial cells co-cultured with trophoblasts. These results indicated that MALAT1 was involved in the pathogenesis of preeclampsia and might be a candidate biomarker as well as a therapeutic target for preeclampsia.






Similar content being viewed by others
Change history
09 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00404-021-06170-z
References
Ananth CV (2014) Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol 38(3):131–132. https://doi.org/10.1053/j.semperi.2014.03.001
Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ (2016) Pre-eclampsia. Lancet 387(10022):999–1011. https://doi.org/10.1016/S0140-6736(15)00070-7
Redman CW (2011) Preeclampsia: a multi-stress disorder. Rev Med Interne 32(Suppl 1):S41-44. https://doi.org/10.1016/j.revmed.2011.03.331
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308(5728):1592–1594. https://doi.org/10.1126/science.1111726
Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30(6):473–482. https://doi.org/10.1016/j.placenta.2009.02.009
Osol G, Moore LG (2014) Maternal uterine vascular remodeling during pregnancy. Microcirculation 21(1):38–47. https://doi.org/10.1111/micc.12080
Pijnenborg R, Vercruysse L, Hanssens M (2006) The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27(9–10):939–958. https://doi.org/10.1016/j.placenta.2005.12.006
Chakraborty C, Gleeson LM, McKinnon T, Lala PK (2002) Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol 80(2):116–124. https://doi.org/10.1139/y02-016
Hannon T, Innes BA, Lash GE, Bulmer JN, Robson SC (2012) Effects of local decidua on trophoblast invasion and spiral artery remodeling in focal placenta accreta—an immunohistochemical study. Placenta 33(12):998–1004. https://doi.org/10.1016/j.placenta.2012.09.004
Wallace AE, Fraser R, Cartwright JE (2012) Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update 18(4):458–471. https://doi.org/10.1093/humupd/dms015
Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D (2007) Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update 13(2):121–141. https://doi.org/10.1093/humupd/dml048
Francis VA, Abera AB, Matjila M, Millar RP, Katz AA (2014) Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells. PLoS ONE 9(6):e99680. https://doi.org/10.1371/journal.pone.0099680
Yang G, Lu X, Yuan L (1839) (2014) LncRNA: a link between RNA and cancer. Biochim Biophys Acta 11:1097–1109. https://doi.org/10.1016/j.bbagrm.2014.08.012
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464(7291):1071–1076. https://doi.org/10.1038/nature08975
Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H (2008) Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 451(7175):202–206. https://doi.org/10.1038/nature06468
Schmidt LH, Spieker T, Koschmieder S, Schaffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, Hillejan L, Wiebe K, Berdel WE, Wiewrodt R, Muller-Tidow C (2011) The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol 6(12):1984–1992. https://doi.org/10.1097/JTO.0b013e3182307eac
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV (2010) The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 39(6):925–938. https://doi.org/10.1016/j.molcel.2010.08.011
Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S (2015) Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med 19(6):1418–1425. https://doi.org/10.1111/jcmm.12576
Xin JW, Jiang YG (2017) Long noncoding RNA MALAT1 inhibits apoptosis induced by oxygen-glucose deprivation and reoxygenation in human brain microvascular endothelial cells. Exp Ther Med 13(4):1225–1234. https://doi.org/10.3892/etm.2017.4095
Kitroser E, Pomeranz M, Epstein Shochet G, Fishman A, Drucker L, Sadeh-Mestechkin D, Lishner M, Tartakover-Matalon S (2012) The involvement of eukaryotic translation initiation factor 4E in extravillous trophoblast cell function. Placenta 33(9):717–724. https://doi.org/10.1016/j.placenta.2012.06.004
Xie H, Zou L, Zhu J, Yang Y (2011) Effects of netrin-1 and netrin-1 knockdown on human umbilical vein endothelial cells and angiogenesis of rat placenta. Placenta 32(8):546–553. https://doi.org/10.1016/j.placenta.2011.04.003
Wang Y, Lewis DF, Gu Y, Zhang Y, Alexander JS, Granger DN (2004) Placental trophoblast-derived factors diminish endothelial barrier function. J Clin Endocrinol Metab 89(5):2421–2428. https://doi.org/10.1210/jc.2003-031707
Schmidt LH, Gorlich D, Spieker T, Rohde C, Schuler M, Mohr M, Humberg J, Sauer T, Thoenissen NH, Huge A, Voss R, Marra A, Faldum A, Muller-Tidow C, Berdel WE, Wiewrodt R (2014) Prognostic impact of Bcl-2 depends on tumor histology and expression of MALAT-1 lncRNA in non-small-cell lung cancer. J Thorac Oncol 9(9):1294–1304. https://doi.org/10.1097/JTO.0000000000000243
Tsujimoto Y, Cossman J, Jaffe E, Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228(4706):1440–1443. https://doi.org/10.1126/science.3874430
Kvansakul M, Hinds MG (2013) Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis 4:e909. https://doi.org/10.1038/cddis.2013.436
Tseng JJ, Hsieh YT, Hsu SL, Chou MM (2009) Metastasis associated lung adenocarcinoma transcript 1 is up-regulated in placenta previa increta/percreta and strongly associated with trophoblast-like cell invasion in vitro. Mol Hum Reprod 15(11):725–731. https://doi.org/10.1093/molehr/gap071
Wong K, Park HT, Wu JY, Rao Y (2002) Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev 12(5):583–591. https://doi.org/10.1016/s0959-437x(02)00343-x
Dickson BJ, Gilestro GF (2006) Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 22:651–675. https://doi.org/10.1146/annurev.cellbio.21.090704.151234
Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, Qian KX, Duan S, Chen Z, Rao Y, Geng JG (2003) Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 4(1):19–29. https://doi.org/10.1016/s1535-6108(03)00164-8
Liao WX, Laurent LC, Agent S, Hodges J, Chen DB (2012) Human placental expression of SLIT/ROBO signaling cues: effects of preeclampsia and hypoxia. Biol Reprod 86(4):111. https://doi.org/10.1095/biolreprod.110.088138
Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, Keshet E (2008) A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res 102(12):1566–1574. https://doi.org/10.1161/CIRCRESAHA.108.171504
Choudhury RH, Dunk CE, Lye SJ, Aplin JD, Harris LK, Jones RL (2017) Extravillous trophoblast and endothelial cell crosstalk mediates leukocyte infiltration to the early remodeling decidual spiral arteriole wall. J Immunol 198(10):4115–4128. https://doi.org/10.4049/jimmunol.1601175
Acknowledgements
We thank the Department of Obstetrics and Gynecology, Union Hospital, Wuhan, for the generous gift of HTR-8/SVneo cell line.
Funding
The work was supported by the National Youth Science Foundation of China (No. 81602863) and Health Commission of Hubei Province Scientific Research Project WJ2019H014.
Author information
Authors and Affiliations
Contributions
FW: project development and manuscript writing. CF: manuscript writing. JJ and XL: data collection. LC: data analysis. All the authors contributed to read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval
All procedures performed in this study involving were in accordance with the ethical standards of the ethics committee of Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (No. 2016S075) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, C., Cheng, L., Jin, J. et al. Long non-coding RNA MALAT1 regulates trophoblast functions through VEGF/VEGFR1 signaling pathway. Arch Gynecol Obstet 304, 873–882 (2021). https://doi.org/10.1007/s00404-021-05987-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-05987-y