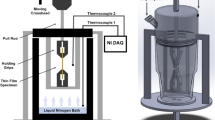
Abstract
A new fixture for the mechanical characterization of thin polymer films under controlled temperature and relative humidity conditions is reported. Novel conducting polymers are often synthesized in small quantities and processed into films on the order of 10–100 microns thick. Standard tensile tests do not allow for adequate testing of these small sample sizes. Hence, a modification of the Sentmanat Extensional Rheometer (SER) to perform tensile testing on thin membranes is presented. Since the standard L-shaped pins do not secure thin polymer films at lower temperatures (i.e., below the melting point), screw down clamps were created to allow for mechanical characterization of solid polymer films. The new testing apparatus allows for mechanical characterization with as little as 2 % of the material needed for testing on a traditional tensile tester. In a parallel effort, a humidity delivery system developed for the TA Instruments ARES-G2 rheometer allows for testing at a range of temperatures (30–100 °C) and relative humidity conditions (0–95 % RH). The novel oven was benchmarked with low density polyethylene and Nafion 115Ⓡ. While the new experiment was built for characterization of ion exchange membranes for fuel cells, the oven is capable of characterizing any environmentally sensitive material using all standard rheometer geometries.













Similar content being viewed by others
References
Ahn S-Y, Lee Y-C, Ha HY, et al. (2004) Properties of the reinforced composite membranes formed by melt soluble ion conducting polymer resins for PEMFCs. Electrochim Acta 50:571–575. doi:10.1016/j.electacta.2004.01.133
ASTM (2012) Standard test method for tensile properties of thin plastic sheeting. ASTM Int D882-12:1–12. doi:10.1520/D0882-12
Baldi F, Franceschini A, Riccò T (2007) Determination of the elongational viscosity of polymer melts by melt spinning experiments. A comparison with different experimental techniques. Rheol Acta 46:965–978. doi:10.1007/s00397-007-0181-z
Bauer F, Denneler S, Willert-Porada M (2005) Influence of temperature and humidity on the mechanical properties of Nafion 117 polymer electrolyte membrane. J Polym Sci Part B Polym Phys 43:786–795. doi:10.1002/polb.20367
Bell GA, Bielinski DM, Beake BD (2008) Influence of water on the nanoindentation creep response of nylon 6. J Appl Polym Sci 107:577–582. doi:10.1002/app
Benziger J, Bocarsly A, Cheah MJ, et al. (2011) Mechanical and transport properties of nafion: effects of temperature and water activity. Struct Bond 141:85–113. doi:10.1007/430
Bhadra S, Kim NH, Choi JS, et al. (2010) Hyperbranched poly(benzimidazole-co-benzene) with honeycomb structure as a membrane for high-temperature proton-exchange membrane fuel cells. J Power Sources 195:2470–2477. doi:10.1016/j.jpowsour.2009.11.083
Borup R, Meyers J, Pivovar B, et al. (2007) Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem Rev 107:3904–51. doi:10.1021/cr050182l
Callister WD (2007) Materials science and engineering: an introduction, 7th edn. Wiley, New York
Choi P, Jalani NH, Thampan TM, Datta R (2006) Consideration of thermodynamic , transport , and mechanical properties in the design of polymer electrolyte membranes for higher temperature fuel cell operation, vol 44, pp 2183–2200. doi:10.1002/polb.20858
DuPont (2009) DuPont Nafion PFSA membranes. Nafion PFSA Membranes
Hadinata C, Boos D, Gabriel C, et al. (2007) Elongation-induced crystallization of a high molecular weight isotactic polybutene-1 melt compared to shear-induced crystallization. J Rheol (N Y N Y) 51:195–215. doi:10.1122/1.2426977
Jalani N, Choi P, Datta R (2005) TEOM: a novel technique for investigating sorption in proton-exchange membranes. J Memb Sci 254:31–38. doi:10.1016/j.memsci.2004.12.020
Jiménez A, Fabra MJ, Talens P, Chiralt A (2012) Edible and biodegradable starch films: a review. Food Bioprocess Technol 5:2058–2076. doi:10.1007/s11947-012-0835-4
Kettle GJ (1977) Variation of the glass transition temperature of nylon-6 with changing water content. Polymer (Guildf) 18:742–743
Kusoglu A, Modestino MA, Hexemer A, et al. (2012a) Subsecond morphological changes in Nafion during water uptake detected by small-angle X-ray scattering. ACS Macro Lett 1:33–36. doi:10.1021/mz200015c
Kusoglu A, Savagatrup S, Clark KT, Weber AZ (2012b) Role of mechanical factors in controlling the structure–function relationship of PFSA ionomers. Macromolecules 45:7467–7476. doi:10.1021/ma301419s
Li Y, Dillard DA, Lai Y-H, et al. (2012) Experimental measurement of stress and strain in Nafion membrane during hydration cycles. J Electrochem Soc 159:B173–B184. doi:10.1149/2.065202jes
Liu Y, Horan J, Schlichting GJ, et al. (2012) A small-angle X-ray Scattering study of the development of morphology in films formed from the 3M perfluorinated sulfonic acid ionomer. Macromolecules 45:7495–7503
Majsztrik PW, Bocarsly AB, Benziger JB (2007) An instrument for environmental control of vapor pressure and temperature for tensile creep and other mechanical property measurements. Rev Sci Instrum 103904:78. doi:10.1063/1.2794736
Martinetti L, Mannion AM, Voje WE, et al. (2014) A critical gel fluid with high extensibility: the rheology of chewing gum. J Rheol (N Y N Y) 58:821–838. doi:10.1122/1.4874322
Merdas I, Thominette F, Tcharkhtchi A, Verdu J (2002) Factors governing water absorption by composite matrices. Compos Sci Technol 62:487–492. doi:10.1016/S0266-3538(01)00138-5
Miri V, Persyn O, Lefebvre J-M, Seguela R (2009) Effect of water absorption on the plastic deformation behavior of nylon 6. Eur Polym J 45:757–762. doi:10.1016/j.eurpolymj.2008.12.008
Müller CMO, Laurindo JB, Yamashita F (2009) Effect of cellulose fibers addition on the mechanical properties and water vapor barrier of starch-based films. Food Hydrocoll 23:1328–1333. doi:10.1016/j.foodhyd.2008.09.002
Olivas GI, Barbosa-Cánovas GV (2008) Alginate–calcium films: water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT - Food Sci Technol 41:359–366. doi:10.1016/j.lwt.2007.02.015
Park J, Kim T-H, Kim HJ, et al. (2012) Crosslinked sulfonated poly(arylene ether sulfone) membranes for fuel cell application. Int J Hydrogen Energy 37:2603–2613. doi:10.1016/j.ijhydene.2011.10.122
Patankar KA, Dillard DA, Case SW, et al. (2012) Linear hygrothermal viscoelastic characterization of Nafion NRE 211 proton exchange membrane. Fuel Cells 12:787–799. doi:10.1002/fuce.201100134
Roberti E, Carlotti G, Cinelli S, et al. (2010) Measurement of the Young’s modulus of Nafion membranes by Brillouin light scattering. J Power Sources 195:7761–7764. doi:10.1016/j.jpowsour.2009.11.033
Sambaer W, Zatloukal M, Kimmer D (2010) The use of novel digital image analysis technique and rheological tools to characterize nanofiber nonwovens. Polym Test 29:82–94. doi:10.1016/j.polymertesting.2009.09.008
Satterfield MB (2007) Mechanical and water sorption properties of Nafion and composite Nafion/titanium dioxide membranes for polymer electrolyte membrane fuel cells. Dissertation, Princeton University
Satterfield MB, Majsztrik PW, Ota H, et al. (2006) Mechanical properties of Nafion and titania/Nafion composite membranes for polymer electrolyte membrane fuel cells. J Polym Sci Part B Polym Phys 44:2327–2345. doi:10.1002/polb
Schlichting GJ, Horan JL, Jessop JD, et al. (2012) A hybrid organic/inorganic ionomer from the copolymerization of vinylphosphonic acid and zirconium vinylphosphonate. Macromolecules 45:3874–3882. doi:10.1021/ma300196y
Sentmanat ML (2004) Miniature universal testing platform?: from extensional melt rheology to solid-state deformation behavior. Rheol Acta:657–669. doi:10.1007/s00397-004-0405-4
Sentmanat ML, Wang BN, McKinley GH (2005) Measuring the transient extensional rheology of polyethylene melts using the SER universal testing platform. J Rheol (N Y N Y) 49:585–606. doi:10.1122/1.1896956
Shi S, Liu D, Liu D, et al. (2014) Mechanical properties and microstructure changes of proton exchange membrane under immersed conditions. Polym Eng Sci 54:2215–2221. doi:10.1002/pen.23770
Stadler FJ, Nishioka A, Stange J, et al. (2007) Comparison of the elongational behavior of various polyolefins in uniaxial and equibiaxial flows. Rheol Acta 46:1003–1012. doi:10.1007/s00397-007-0190-y
Tang Y, Karlsson AM, Santare MH, et al. (2006) An experimental investigation of humidity and temperature effects on the mechanical properties of perfluorosulfonic acid membrane. Mater Sci Eng A 425:297–304. doi:10.1016/j.msea.2006.03.055
Thompson DG, Osborn JC, Kober EM, Schoonover JR (2006) Effects of hydrolysis-induced molecular weight changes on the phase separation of a polyester polyurethane. Polym Degrad Stab 91:3360–3370. doi:10.1016/j.polymdegradstab.2006.05.019
Vandiver MA, Caire BR, Carver JR, et al. (2014) Mechanical characterization of anion exchange membranes by extensional rheology under controlled hydration. J Electrochem Soc 161:H677–H683. doi:10.1149/2.0971410jes
Wang Y, Wang S-Q (2008) From elastic deformation to terminal flow of a monodisperse entangled melt in uniaxial extension. J Rheol (N Y N Y) 52:1275–1290. doi:10.1122/1.2995858
Weber AZ, Newman J (2003) Transport in polymer-electrolyte membranes. J Electrochem Soc 150:A1008. doi:10.1149/1.1580822
White CC, Hunston DL, Tan KT, et al. (2013) An accelerated exposure and testing apparatus for building joint sealants. Rev Sci Instrum 095113:84. doi:10.1063/1.4821880
Zawodzinski TA, Springer TE, Davey J, et al. (1993) A comparative study of water uptake by and transport through ionomeric fuel cell membranes. J Electrochem Soc 140:1981–1985
Acknowledgments
The authors thank the U.S. Army Research Office (MURI Grant No. W911NF-10-1-0520 and DURIP Grant No. W911NF-11-1-0306) for its support of this work. The authors thank Aadil Elmoumni of TA Instruments for the useful discussions. The authors thank Mountain States Plastics for the samples of LDPE/LLDPE blown films they provided. The authors thank Kurt Johnson and the whole team at Challenger Manufacturing Consultants for their expertise in designing and building the modified SER drums and the oven. The authors thank Jessica Earl (NSF sponsored REU student) for the early modeling work on the transport dynamics in the oven.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Caire, B.R., Vandiver, M.A. & Liberatore, M.W. Mechanical testing of small, thin samples in a humidity-controlled oven. Rheol Acta 54, 253–261 (2015). https://doi.org/10.1007/s00397-014-0834-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-014-0834-7