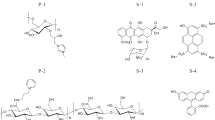
Abstract
In this paper, we describe three series of polyampholytes synthesized via quaternization of poly(4-vinylpyridin) by ω-bromocarboxylic acids and alkyl bromides: (1) with cationic and anionic groups in each unit (polybetaines), (2) with betaine and cationic groups, and (3) with betaine and pendant alkyl groups. The polymers were complexed with anionic mixed lipid membranes, liposomes, and Langmuir monolayers. By varying a length of –(CH2)n– spacer in the betaine group, different behaviors of polybetaines in a suspension of anionic liposomes can be realized: from no interaction to complexation followed by significant structural reorganization in the liposomal membrane. Cytotoxicities of polyampholytes are one to two orders of magnitude less than the cytotoxicity of a pure polycationic polymer with the same degree of polymerization. These results are of importance in designing polyelectrolytes with a higher affinity to the biolodical (cell) membrane and minimum cytotoxicity and demonstrate the potential of polyampholytes in developing biocompatible polymeric structures.













Similar content being viewed by others
References
Qin T, Yin Y, Huang L, Yu Q, Yang Q (2015) H9N2 influenza whole inactivated virus combined with polyethyleneimine strongly enhances mucosal and systemic immunity after intranasal immunization in mice. Clin Vaccine Immunol 22(4):421–429. doi:10.1128/CVI.00778-14
Chen X, Wang L, Liu Q, Jia J, Liu Y, Zhang W, Su Z (2014) Polycation-decorated PLA microspheres induce robust immune responses via commonly used parenteral administration routes. Int Immunopharmacol 23(2):592–602. doi:10.1016/j.intimp.2014.10.010
Sheppard NC, Brinckmann SA, Gartlan KH, Puthia M, Svanborg C, Krashias G, Wegmann F (2014) Polyethyleneimine is a potent systemic adjuvant for glycoprotein antigens. Int Immunopharmacol 26(10):531–538. doi:10.1093/intimm/dxu055
Dyakonova VA, Dambaeva SV, Pinegin BV, Khaitov RM (2004) Study of interaction between the polyoxidonium immunomodulator and the human immune system cells. Int Immunopharmacol 4(13):1615–1623. doi:10.1016/j.intimp.2004.07.015
Zarubina IV, Shabanov PD (2015) Antioxidant effect of polyoxidonium and metaprot during bronchopulmonary inflammation in rats. Bull Exp Biol Med 160(2):234–237. doi:10.1007/s10517-015-3137-9
Hoque J, Akkapeddi P, Ghosh C, Uppu DSSM, Haldar J (2016) A biodegradable polycationic paint that kills bacteria in vitro and in vivo. ACS Appl Mater Inter 8(43):29298–29309. doi:10.1021/acsami.6b09804
Yin M, Li Z, Zhou L, Dong K, Ren J, Qu X (2016) A multifunctional upconverting nanoparticle incorporated polycationic hydrogel for near-infrared triggered and synergistic treatment of drug-resistant bacteria. Nanotechnology 27(12):125601. doi:10.1088/0957-4484/27/12/125601
Kim Y-H, Kim SM, Lee SY (2015) Antimicrobial activity of protamine against oral microorganisms. Biocontrol Sci Techn 20(4):275–280. doi:10.4265/bio.20.275
Atar-Froyman L, Sharon A, Weiss EI, Houri-Haddad Y, Kesler-Shvero D, Domb AJ, Beyth N (2015) Anti-biofilm properties of wound dressing incorporating nonrelease polycationic antimicrobials. Biomaterials 46:141–148. doi:10.1016/j.biomaterials.2014.12.047
Kasyanenko N, Bakulev V, Perevyazko I, Nekrasova T, Nazarova O, Slita A, Panarin E (2016) Model system for multifunctional delivery nanoplatforms based on DNA-polymer complexes containing silver nanoparticles and fluorescent dye. J Biotech 236:78–87. doi:10.1016/j.jbiotec.2016.08.010
Albuquerque LJ, Annes K, Milazzotto MP, Mattei B, Riske KA, Jäger E, Pánek J, Štěpánek P, Kapusta P, Muraro PI, De Freitas AG, Schmidt V, Giacomelli C, Bonvent JJ, Giacomelli FC (2016) Efficient condensation of DNA into environmentally responsive polyplexes produced from block catiomers carrying amine or diamine groups. Langmuir 32(2):577–586. doi:10.1021/acs.langmuir.5b04080
Sardo C, Craparo EF, Porsio B, Giammona G, Cavallaro G (2016) Improvements in rational design strategies of inulin derivative polycation for siRNA delivery. Biomacromolecules 17(7):2352–2366. doi:10.1021/acs.biomac.6b00281
Hrubý M, Filippov SK, Štěpánek P (2015) Smart polymers in drug delivery systems on crossroads: which way deserves following? Eur Polym J 65:82–97. doi:10.1016/j.eurpolymj.2015.01.016
Jäger A, Jäger E, Surman F, Höcherl A, Angelov B, Ulbrich K, Štěpánek P (2015) Nanoparticles of the poly([N-(2-hydroxypropyl)]methacrylamide)-b-poly[2-(diisopropylamino)ethyl methacrylate] diblock copolymer for pH-triggered release of paclitaxel. Polym Chem 6(27):4946–4954. doi:10.1039/C5PY00567A
Cheow WS, Hadinoto K (2012) Green amorphous nanoplex as a new supersaturating drug delivery system. Langmuir 28(15):6265–6275. doi:10.1021/la204782x
Muzzio NE, Pasquale MA, Gregurec D, Diamanti E, Kosutic M, Azzaroni O, Moya SE (2016) Polyelectrolytes multilayers to modulate cell adhesion: a study of the influence of film composition and polyelectrolyte interdigitation on the adhesion of the A549 cell line. Macromol Biosci 16(4):482–495. doi:10.1002/mabi.201500275
Zhang B-B, Wang L, Charles V, Rooke JC, Su B-L (2016a) Robust and biocompatible hybrid matrix with controllable permeability for microalgae encapsulation. ACS Appl Mater Inter 8(14):8939–8946. doi:10.1021/acsami.6b00191
Tam SK, Bilodeau S, Dusseault J, Langlois G, Hallé J-P, Yahia LH (2011) Biocompatibility and physicochemical characteristics of alginate–polycation microcapsules. Acta Biomater 7(4):1683–1692. doi:10.1016/j.actbio.2010.12.006
Guo M, Yan Y, Liu X, Yan H, Liu K, Zhang H, Cao Y (2010) Multilayer nanoparticles with a magnetite core and a polycation inner shell as pH-responsive carriers for drug delivery. Nanoscale 2(3):434–441. doi:10.1039/B9NR00244H
Zhang L, Zhang Y, Chen Z, He Y (2016b) Intracellular redox-responsive nanocarrier for plasmid delivery: in vitro characterization and in vivo studies in mice. Int J Nanomedicine 11:5245–5256. doi:10.2147/IJN.S94995
Wang M, Wu B, Tucker JD, Lu P, Lu Q (2015) Cationic polyelectrolyte-mediated delivery of antisense morpholino oligonucleotides for exon-skipping in vitro and in mdx mice. Int J Nanomedicine 5635. doi:10.2147/IJN.S89910
Tang Y, Han S, Liu H, Chen X, Huang L, Li X, Zhang J (2013) The role of surface chemistry in determining in vivo biodistribution and toxicity of CdSe/ZnS core–shell quantum dots. Biomaterials 34(34):8741–8755. doi:10.1016/j.biomaterials.2013.07.087
Akagi D, Oba M, Koyama H, Nishiyama N, Fukushima S, Miyata T, Kataoka K (2007) Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther 14(13):1029–1038. doi:10.1038/sj.gt.3302945
Yaroslavov AA, Zaborova OV, Sybachin AV, Kalashnikova IV, Kesselman E, Schmidt E, Talmon E, Rodriguez AR, Deming TJ (2015a) Biodegradable containers composed of anionic liposomes and cationic polypeptide vesicles. RSC Adv 5(119):98687–98691. doi:10.1039/C5RA15863J
Yaroslavov AA, Sybachin AV, Zaborova OV, Pergushov DV, Zezin AB, Melik-Nubarov NS, Plamper FA, Müller AHE, Menger FM (2014) Electrostatically driven complexation of liposomes with a star-shaped polyelectrolyte to low-toxicity multi-liposomal assemblies. Macromol Biosci 14(4):491–495. doi:10.1002/mabi.201300436
Cai J, Yue Y, Wang Y, Jin Z, Jin F, Wu C (2016) Quantitative study of effects of free cationic chains on gene transfection in different intracellular stages. J Control Release 238:71–79. doi:10.1016/j.jconrel.2016.07.031
Peng Q, Zhu J, Yu Y, Hoffman L, Yang X (2015) Hyperbranched lysine−arginine copolymer for gene delivery. J Biomat Sci 26(16):1163–1177. doi:10.1080/09205063.2015.1080482
Alhakamy NA, Berkland CJ (2013) Polyarginine molecular weight determines transfection efficiency of calcium condensed complexes. Mol Pharm 10(5):1940–1948. doi:10.1021/mp3007117
Salmasi Z, Shier WT, Hashemi M, Mahdipour E, Parhiz H, Abnous K, Ramezani M (2015) Heterocyclic amine-modified polyethylenimine as gene carriers for transfection of mammalian cells. Eur J Pharm Biopharm 96:76–88. doi:10.1016/j.ejpb.2015.07.008
Han HM, Gopal R, Park Y (2016) Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties. Amino Acids 48(2):505–522. doi:10.1007/s00726-015-2104-0
Hall A, Parhamifar L, Lange MK, Meyle KD, Sanderhoff M, Andersen H, Moghimi SM (2015) Polyethylenimine architecture-dependent metabolic imprints and perturbation of cellular redox homeostasis. Biochim Biophys Acta 1847(3):328–342. doi:10.1016/j.bbabio.2014.12.002
Flebus L, Lombart F, Martinez-Jothar L, Sevrin C, Delierneux C, Oury C, Grandfils C (2015) In vitro study of the specific interaction between poly(2-dimethylamino ethylmethacrylate) based polymers with platelets and red blood cells. Int J Pharm 492(1):55–64. doi:10.1016/j.ijpharm.2015.06.036
Yaroslavov AA, Efimova AA, Sybachin AV (2009a) Effect of the phase state of the lipid bilayer on the structure and characteristics of the polycation-(anionic liposome) complex. Polym Sci Ser A 51(6):638–647. doi:10.1134/S0965545X0906008X
Efimova AA, Sybachin AV, Yaroslavov AA (2011) Effect of anionic-lipid-molecule geometry on the structure and properties of liposome-polycation complexes. Polym Sci Ser C 53(1):89–96. doi:10.1134/S1811238211040011
Angelov B, Angelova A, Filippov SK, Drechsler M, Štěpánek P, Lesieur S (2014) Multicompartment lipid cubic nanoparticles with high protein upload: millisecond dynamics of formation. ACS Nano 8:5216–5226. doi:10.1021/nn5012946
Yaroslavov AA, Sybachin AV, Kesselman E, Schmidt J, Talmon Y, Rizvi SAA, Menger FM (2011a) Liposome fusion rates depend upon the conformation of polycation catalysts. J Am Chem Soc 133(9):2881–2883. doi:10.1021/ja111406q
Schulz M, Olubummo A, Binder WH, Ringsdorf H, Schlarb B, Venzmer J, Schanze KS (2012) Beyond the lipid-bilayer: interaction of polymers and nanoparticles with membranes. Soft Matter 8(18):4849–4864. doi:10.1039/c2sm06999g
Wang C, Zolotarskaya OY, Nair SS, Ehrhardt CJ, Ohman DE, Wynne KJ, Yadavalli VK (2016) Real-time observation of antimicrobial polycation effects on Escherichia coli: adapting the carpet model for membrane disruption to quaternary copolyoxetanes. Langmuir 32(12):2975–2984. doi:10.1021/acs.langmuir.5b04247
Haas H, Steitz R, Fasano A, Liuzzi GM, Polverini E, Cavatorta P, Riccio P (2007) Laminar order within Langmuir−Blodgett multilayers from phospholipid and myelin basic protein: a neutron reflectivity study. Langmuir 23(16):8491–8496. doi:10.1021/la700733y
Robison AD, Sun S, Poyton MF, Johnson GA, Pellois J-P, Jungwirth P, Cremer PS (2016) Polyarginine interacts more strongly and cooperatively than polylysine with phospholipid bilayers. J Phys Chem B 120(35):9287–9296. doi:10.1021/acs.jpcb.6b05604
Yaroslavov AA, Sitnikova TA, Rakhnyanskaya AA, Yaroslavova EG, Davydov DA, Burova TV, Menger FM (2009b) Biomembrane sensitivity to structural changes in bound polymers. J Am Chem Soc 131(5):1666–1667. doi:10.1021/ja808461s
Sybachin AV, Zaborova OV, Ballauff M, Kesselman E, Schmidt J, Talmon Y, Menger FM, Yaroslavov AA (2012) Composition and properties of complexes between spherical polycationic brushes and anionic liposomes. Langmuir 28(46):16108–16114. doi:10.1021/la3024265
Yaroslavov AA, Sitnikova TA, Rakhnyanskaya AA, Ermakov YA, Burova TV, Grinberg VYA, Menger FM (2007) Contrasting behavior of zwitterionic and cationic polymers bound to anionic liposomes. Langmuir 23(14):7539–7544. doi:10.1021/LA700637D
Szilagyi I, Trefalt G, Tiraferri A, Maroni P, Borkovec M, Bolto B, Richmond P (2014) Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 10(15):2479–2502. doi:10.1039/c3sm52132j
Sybachin AV, Zaborova OV, Pergushov DV, Zezin AB, Plamper FA, Müller AHE, Kesselman E, Schmidt E, Talmon Y, Menger FM, Yaroslavov AA (2016) Complexes of star-shaped cationic polyelectrolytes with anionic liposomes: towards multi-liposomal assemblies with controllable stability. Polymer 93:198–203. doi:10.1016/j.polymer.2016.04.025
Yaroslavov AA, Sybachin AV, Zaborova OV, Migulin VA, Samoshin VV, Ballauff M, Menger FM (2015b) Capacious and programmable multi-liposomal carriers. Nanoscale 7(5):1635–1641. doi:10.1039/C4NR06037G
Ivashkov OV, Sybachin AV, Efimova AA, Pergushov DV, Orlov VN, Schmalz H, Yaroslavov AA (2015) The influence of the chain length of polycations on their complexation with anionic liposomes. ChemPhysChem 16(13):2849–2853. doi:10.1002/cphc.201500474
Sivov NA (2006) In: Biocide guanidine containing polymers: synthesis, structure and properties. CRC Press
Thomas TP, Majoros I, Kotlyar A, Mullen D, Holl MM, Baker Jr JR (2009) Cationic poly(amidoamine) dendrimer induces lysosomal apoptotic pathway at therapeutically relevant concentrations. Biomacromolecules 10(12):3207–3214. doi:10.1021/bm900683r
Kwolek U, Jamróz D, Janiczek M, Nowakowska M, Wydro P, Kepczynski M (2016) Interactions of polyethylenimines with zwitterionic and anionic lipid membranes. Langmuir 32(19):5004–5018. doi:10.1021/acs.langmuir.6b00490
Yaroslavov AA, Sybachin AV, Zaborova OV, Orlov VN, Ballauff M, Talmon Y, Menger FM (2013) Lipid segregation in membranes of anionic liposomes adsorbed onto polycationic brushes. Chem Eur J 19(41):13674–13678. doi:10.1002/chem.201301944
Sybachin AV, Zaborova OV, Orlov VN, Semenyuk PI, Ballauff M, Kesselman E, Schmidt J, Talmon Y, Menger FM, Yaroslavov AA (2014) Complexes between anionic liposomes and spherical polycationic brushes. An assembly of assemblies. Langmuir 30(9):2441–2447. doi:10.1021/la4036248
Ciumac D, Campbell RA, Xu H, Clifton LA, Hughes AV, Webster JRP, Lu JR (2016) Implications of lipid monolayer charge characteristics on their selective interactions with a short antimicrobial peptide. Colloids Surf B Biointerfaces. doi:10.1016/j.colsurfb.2016.10.043
Pazin WM, Olivier DS, Vilanova N, Ramos AP, Voets IK, Soares AEE, Ito AS (2016) Interaction of Artepillin C with model membranes. Eur Biophys J. doi:10.1007/s00249-016-1183-5
Kurniawan J, Suga K, Kuhl TL (2016) Interaction forces and membrane charge tunability: oleic acid containing membranes in different pH conditions. Biochim Biophys Acta. doi:10.1016/j.bbamem.2016.11.001
Dékány G, Csóka I, Erös I (2001) Interaction between liposomes and neutral polymers: effect of adsorption on drug release György Dékány, Ildikó Csóka & István Erös interaction between liposomes and neutral polymers: effect of adsorption on drug release. J Disper Sci Technol 22(5):461–472. doi:10.1081/DIS-100107855
Leitmannova Liu A. (2008) In: Advances in planar lipid bilayers and liposomes. Elsevier
Xie AF, Granick S (2002) Phospholipid membranes as substrates for polymer adsorption. Nat Mater 1:129–133. doi:10.1038/nmat738
Kučerka N, Nieh M-P, Katsaras J (2011) Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim Biophys Acta 1808(11):2761–2771. doi:10.1016/j.bbamem.2011.07.022
Sennato S, Carlini L, Truzzolillo D, Bordi F (2016) Salt-induced reentrant stability of polyion-decorated particles with tunable surface charge density. Colloids Surf B Biointerfaces 137:109–120. doi:10.1016/j.colsurfb.2015.06.011
Yaroslavov AA, Melik-Nubarov NS, Menger FM (2006) Polymer-induced flip-flop in biomembranes. Acc Chem Res 39(10):702–710. doi:10.1021/ar050078q
Sybachin AV, Efimova AA, Litmanovich EA, Menger FM, Yaroslavov AA (2007) Complexation of polycations to anionic liposomes: composition and structure of the interfacial complexes. Langmuir 23(20):10034–10039. doi:10.1021/la701411y
Yaroslavov AA, Efimova AA, Sybachin AV, Izumrudov VA, Samoshin VV, Potemkin II (2011b) Stability of anionic liposome-cationic polymer complexes in water-salt media. Colloid J 73(3):430–435. doi:10.1134/S1061933X11030185
Sitnikova TA, Rakhnyanskaya AA, Yaroslavova EG, Melik-Nubarov NS, Yaroslavov AA (2013) Physicochemical and biological properties of polyampholytes: quaternized derivatives of poly(4-vinylpyridine). Polym Sci Ser A 55(3):163–170. doi:10.1134/S0965545X13030061
Fischer, D. et al (2003) In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24(7):1121–31. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12527253. Accessed 27 Nov 2016.
Qi P, Bu Y, Xu J, Qin B, Luan S, Song S (2016) pH-responsive release of paclitaxel from hydrazone-containing biodegradable micelles. Colloid Polym Sci. doi:10.1007/s00396-016-3968-6
Yaroslavov AA, Sybachin AV, Zaborova OV, Zezin AB, Talmon Y, Ballauff M, Menger FM (2015c) Multi-liposomal containers. Adv Colloid Interf Sci 226:54–64. doi:10.1016/j.cis.2015.08.011
Acknowledgements
This work was supported by the Russian Science Foundation (project 14-13-00255).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yaroslavov, A.A., Sitnikova, T.A., Rakhnyanskaya, A.A. et al. Variable and low-toxic polyampholytes: complexation with biological membranes. Colloid Polym Sci 295, 1405–1417 (2017). https://doi.org/10.1007/s00396-017-4054-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4054-4