Abstract
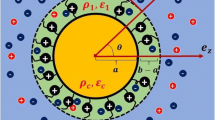
Measurement and analysis of electrophoretic mobility (EPM) are widely used to investigate electric charging properties of proteins. However, the proper way of analysis for EPM of protein has not yet been fully consolidated. In this study, the EPM of hen-egg-white lysozyme (LSZ) was measured as a function of pH at different concentrations of KCl solutions. The obtained experimental EPMs are compared to theoretical EPMs which were calculated from charge amount from proton titration. Theoretical EPMs were calculated by a set of models for a small rigid particle including Poisson-Boltzmann model and the effect of double-layer relaxation and by that for a soft particle neglecting the relaxation effect. The results of comparisons show that one can analyze the EPM of LSZ as a small rigid particle. Nevertheless, all analyses overestimate experimental data. We presume that these discrepancies are caused by the shift of slipping plane from the surface and/or by binding of counterion to LSZ. Therefore, we examined these two effects on the analyses of EPM. Our analyses demonstrate that introducing the 0.5–2 nm shift of slipping plane or the 40–80 % reduction of effective charge generates the quantitative agreement between theoretical EPMs and experimental data. We find the required amount of reduced charge is 4–5 elementary charges per LSZ irrespective of pH and ionic strength below pH 7.







Similar content being viewed by others
References
Štajner L, Požar J, Kovačević D (2015) Complexation between lysozyme and sodium poly(styrenesulfonate): the effect of pH, reactant concentration and titration direction. Colloids Surfaces A Physicochem. Eng Asp 483:171–180. doi:10.1016/j.colsurfa.2015.03.034
Su T, Lu J, Thomas R, Cui Z, Penfold J (1998) The adsorption of lysozyme at the silica-water interface: a neutron reflection study. J Colloid Interface Sci 203:419–429. doi:10.1006/jcis.1998.5545
Sofińska K, Adamczyk Z, Kujda M, Nattich-Rak M (2014) Recombinant albumin monolayers on latex particles. Langmuir 30:250–258. doi:10.1021/la403715s
Norde W, Gonzalez FG, Haynes CA (1995) Protein adsorption on polystyrene latex particles. Polym Adv Technol 6:518–525. doi:10.1002/pat.1995.220060713
Bharti B, Meissner J, Findenegg GH (2011) Aggregation of silica nanoparticles directed by adsorption of lysozyme. Langmuir 27:9823–9833. doi:10.1021/la201898v
Wang Z, Gong X, Ngai T (2015) Measurements of long-range interactions between protein-functionalized surfaces by Total internal reflection microscopy. Langmuir 31:3101–3107. doi:10.1021/acs.langmuir.5b00090
Smoluchowski M (1903) Contribution à la théorie l’endosmose électrique et de quelques phénomènes corrélatifs. Bull. Int. l’Académie Des Sci. Cracovie, Cl. Des Sci. Mathématiques Nat 8:182–199
Huckel E (1924) Die kataphorese der kugel. Phys Z 25:204–210
Henry DC (1931) The cataphoresis of suspended particles, part 1. The equation of cataphoresis. Proc R Soc L 133A:106–129
O’Brien RW, White LR (1978) Electrophoretic mobility of a spherical colloidal particle. J Chem Soc Faraday Trans 2 74:1607–1626. doi:10.1039/f29787401607
Ohshima H, Healy TW, White LR (1983) Approximate analytic expressions for the electrophoretic mobility of spherical colloidal particles and the conductivity of their dilute suspensions. J Chem Soc Faraday Trans 2 79:1613–1628. doi:10.1039/f29837901613
Ohshima H (2001) Approximate analytic expression for the electrophoretic mobility of a spherical colloidal particle. J Colloid Interface Sci 239:587–590. doi:10.1006/jcis.2001.7608
Kobayashi M, Skarba M, Galletto P, Cakara D, Borkovec M (2005) Effects of heat treatment on the aggregation and charging of Stober-type silica. J Colloid Interface Sci 292:139–147. doi:10.1016/j.jcis.2005.05.093
Čop A, Kovačević D, Dragić T, Kallay N (2003) Evaluation of equilibrium parameters characterizing metal oxide/electrolyte solution interface. Colloids Surfaces A Physicochem. Eng. Asp. 230:159–165. doi:10.1016/j.colsurfa.2003.09.022
Sugimoto T, Kobayashi M, Adachi Y (2014) The effect of double layer repulsion on the rate of turbulent and Brownian aggregation: experimental consideration. Colloids Surfaces A Physicochem. Eng. Asp. 443:418–424. doi:10.1016/j.colsurfa.2013.12.002
Borkovec M, Behrens SH, Semmler M (2000) Observation of the mobility maximum predicted by the standard electrokinetic model for highly charged amidine latex particles. Langmuir 16:5209–5212. doi:10.1021/la9916373
Kobayashi M (2008) Electrophoretic mobility of latex spheres in the presence of divalent ions: experiments and modeling. Colloid Polym Sci 286:935–940. doi:10.1007/s00396-008-1851-9
Chassagne C, Ibanez M (2012) Electrophoretic mobility of latex nanospheres in electrolytes: experimental challenges. Pure Appl Chem 85(1):41–51. doi:10.1351/PAC-CON-12-02-12
Kobayashi M, Sasaki A (2014) Electrophoretic mobility of latex spheres in mixture solutions containing mono and divalent counter ions. Colloids Surfaces A Physicochem Eng Asp 440:74–78. doi:10.1016/j.colsurfa.2012.10.036
Antonietti M, Vorwerg L (1997) Examination of the atypical electrophoretic mobility behavior of charged colloids in the low salt region using the O’Brian-White theory. Colloid Polym Sci 275:883–887. doi:10.1007/s003960050161
Harding IH, Healy TW (1985) Electrical double layer properties of amphoteric polymer latex colloids. J Colloid Interface Sci 107:382–397. doi:10.1016/0021-9797(85)90191-2
Delgado AV, González-Caballero F, Hunter RJ, Koopal LK, Lyklema J (2007) Measurement and interpretation of electrokinetic phenomena. J Colloid Interface Sci 309:194–224. doi:10.1016/j.jcis.2006.12.075
Lin W, Galletto P, Borkovec M (2004) Charging and aggregation of latex particles by oppositely charged dendrimers. Langmuir 20:7465–7473. doi:10.1021/la049006i
Jachimska B, Kozlowska A, Pajor-Swierzy A (2012) Protonation of lysozymes and its consequences for the adsorption onto a mica surface. Langmuir 28:11502–11510. doi:10.1021/la301558u
Gokarn YR, Fesinmeyer RM, Saluja A, Razinkov V, Chase SF, Laue TM, et al. (2011) Effective charge measurements reveal selective and preferential accumulation of anions, but not cations, at the protein surface in dilute salt solutions. Protein Sci 20:580–587. doi:10.1002/pro.591
Tan WF, Norde W, Koopal LK (2011) Humic substance charge determination by titration with a flexible cationic polyelectrolyte. Geochim Cosmochim Acta 75:5749–5761. doi:10.1016/j.gca.2011.07.015
Kim JY, Ahn SH, Kang ST, Yoon BJ (2006) Electrophoretic mobility equation for protein with molecular shape and charge multipole effects. J Colloid Interface Sci 299:486–492. doi:10.1016/j.jcis.2006.02.003
Allison SA, Potter M, McCammon JA (1997) Modeling the electrophoresis of lysozyme. II. Inclusion of ion relaxation. Biophys J 73:133–140. doi:10.1016/S0006-3495(97)78054-8
Kuehner DE, Engmann J, Fergg F, Wernick M, Blanch HW, Prausnitz JM (1999) Lysozyme net charge and ion binding in concentrated aqueous electrolyte solutions. J Phys Chem B 103:1368–1374. doi:10.1021/jp983852i
Tan WF, Koopal LK, Weng LP, van Riemsdijk WH, Norde W (2008) Humic acid protein complexation. Geochim Cosmochim Acta 72:2090–2099. doi:10.1016/j.gca.2008.02.009
Lundin M, Macakova L, Dedinaite A, Claesson P (2008) Interactions between chitosan and SDS at a low-charged silica substrate compared to interactions in the bulk—the effect of ionic strength. Langmuir 24:3814–3827. doi:10.1021/la702653m
Makino K, Ohshima H (2010) Electrophoretic mobility of a colloidal particle with constant surface charge density. Langmuir 26:18016–18019. doi:10.1021/la1035745
Ohshima H, Healy TW, White LR (1982) Accurate analytic expressions for the surface charge density/surface potential relationship and double-layer potential distribution for a spherical colloidal particle. J Colloid Interface Sci 90:17–26. doi:10.1016/0021-9797(82)90393-9
Ohshima H (1994) A simple expression for Henry’s function for the retardation effect in electrophoresis of spherical colloidal particles. J Colloid Interface Sci 168:269–271. doi:10.1006/jcis.1994.1419
Hermans JJ, Fujita H (1955) Electrophoresis of charged polymer molecules with partial free drainage. Proc. K. Ned. Akad. Wet., Ser. B Phys. Sci. 58
Ohshima H (1996) Henry’s function for electrophoresis of a cylindrical colloidal particle. J Colloid Interface Sci 180:299–301. doi:10.1006/jcis.1996.0305
Acknowledgments
This study was financially supported by the KAKENHI (15H04563) from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 272 kb)
Rights and permissions
About this article
Cite this article
Yamaguchi, A., Kobayashi, M. Quantitative evaluation of shift of slipping plane and counterion binding to lysozyme by electrophoresis method. Colloid Polym Sci 294, 1019–1026 (2016). https://doi.org/10.1007/s00396-016-3852-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3852-4