Abstract
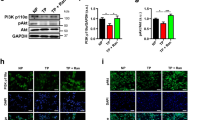
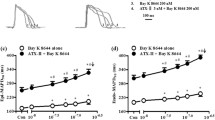
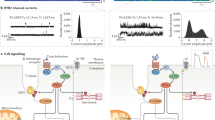
Atrial fibrillation (AF) has been associated with increased spontaneous calcium release from the sarcoplasmic reticulum and linked to increased adenosine A2A receptor (A2AR) expression and activation. Here we tested whether this may favor atrial arrhythmogenesis by promoting beat-to-beat alternation and irregularity. Patch-clamp and confocal calcium imaging was used to measure the beat-to-beat response of the calcium current and transient in human atrial myocytes. Responses were classified as uniform, alternating or irregular and stimulation of Gs-protein coupled receptors decreased the frequency where a uniform response could be maintained from 1.0 ± 0.1 to 0.6 ± 0.1 Hz; p < 0.01 for beta-adrenergic receptors and from 1.4 ± 0.1 to 0.5 ± 0.1 Hz; p < 0.05 for A2ARs. The latter was linked to increased spontaneous calcium release and after-depolarizations. Moreover, A2AR activation increased the fraction of non-uniformly responding cells in HL-1 myocyte cultures from 19 ± 3 to 51 ± 9 %; p < 0.02, and electrical mapping in perfused porcine atria revealed that adenosine induced electrical alternans at longer cycle lengths, doubled the fraction of electrodes showing alternation, and increased the amplitude of alternations. Importantly, protein kinase A inhibition increased the highest frequency where uniform responses could be maintained from 0.84 ± 0.12 to 1.86 ± 0.11 Hz; p < 0.001 and prevention of A2AR-activation with exogenous adenosine deaminase selectively increased the threshold from 0.8 ± 0.1 to 1.2 ± 0.1 Hz; p = 0.001 in myocytes from patients with AF. In conclusion, A2AR-activation promotes beat-to-beat irregularities in the calcium transient in human atrial myocytes, and prevention of A2AR activation may be a novel means to maintain uniform beat-to-beat responses at higher beating frequencies in patients with atrial fibrillation.








Similar content being viewed by others
References
Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, Wasserstrom JA (2006) Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res 99:e65–e73. doi:10.1161/01.RES.0000244087.36230.bf
Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O (2006) Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation 114:2434–2442. doi:10.1161/CIRCULATIONAHA.106.633735
Bertolet BD, Hill JA, Kerensky RA, Belardinelli L (1997) Myocardial infarction related atrial fibrillation: role of endogenous adenosine. Heart 78:88–90
Bitigen A, Bulut M, Tanalp AC, Kirma C, Barutcu I, Pala S, Erkol A, Boztosun B (2007) Left atrial appendage functions in patients with severe rheumatic mitral regurgitation. Int J Cardiovasc Imaging 23:693–700. doi:10.1007/s10554-007-9207-y
Bolter CP, Atkinson KJ (1988) Influence of temperature and adrenergic stimulation on rat sinoatrial frequency. Am J Physiol 254:R840–R844
Burashnikov A, Antzelevitch C (2003) Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 107:2355–2360. doi:10.1161/01.CIR.0000065578.00869.7C
Chang KC, Bayer JD, Trayanova NA (2014) Disrupted calcium release as a mechanism for atrial alternans associated with human atrial fibrillation. PLoS Comput Biol 10:e1004011. doi:10.1371/journal.pcbi.1004011
Cinca J, Sassine A, Deceuninck P, Roca J, Gagne P, Morena H, Puech P (1978) The dependence of T wave alternans on diastolic resting period duration. Eur J Cardiol 7:299–309
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105. doi:10.1042/bj20021535
Diaz ME, O’Neill SC, Eisner DA (2004) Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res 94:650–656. doi:10.1161/01.RES.0000119923.64774.72
Dinanian S, Boixel C, Juin C, Hulot JS, Coulombe A, Rucker-Martin C, Bonnet N, Le Grand B, Slama M, Mercadier JJ, Hatem SN (2008) Downregulation of the calcium current in human right atrial myocytes from patients in sinus rhythm but with a high risk of atrial fibrillation. Eur Heart J 29:1190–1197. doi:10.1093/eurheartj/ehn140
El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D (2006) Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation 114:670–680. doi:10.1161/CIRCULATIONAHA.106.636845
Florea SM, Blatter LA (2012) Regulation of cardiac alternans by beta-adrenergic signaling pathways. Am J Physiol Heart Circ Physiol 303:H1047–H1056. doi:10.1152/ajpheart.00384.2012
Glasscock E, Voigt N, McCauley M, Sun Q, Li N, Chiang D, Zhou X-B, Molina C, Thomas D, Schmidt C, Skapura D, Noebels J, Dobrev D, Wehrens XT (2015) Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Basic Res Cardiol C7–47(110):1–15. doi:10.1007/s00395-015-0505-6
Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB, Christini DJ (2007) Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: a simulation study. Circulation 115:2094–2102. doi:10.1161/CIRCULATIONAHA.106.656504
Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, Ohyanagi M (2005) Discordant repolarization alternans-induced atrial fibrillation is suppressed by verapamil. Circ J 69:1368–1373 (JST.JSTAGE/circj/69.1368 [pii])
Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J (2004) Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 110:1358–1363. doi:10.1161/01.CIR.0000141296.59876.87
Hove-Madsen L, Prat-Vidal C, Llach A, Ciruela F, Casado V, Lluis C, Bayes-Genis A, Cinca J, Franco R (2006) Adenosine A2A receptors are expressed in human atrial myocytes and modulate spontaneous sarcoplasmic reticulum calcium release. Cardiovasc Res 72:292–302. doi:10.1016/j.cardiores.2006.07.020
Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA (2000) Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol 524(Pt 3):795–806. doi:10.1111/j.1469-7793.2000.00795.x
Hussain M, Orchard CH (1997) Sarcoplasmic reticulum Ca2+ content, l-type Ca2+ current and the Ca2+ transient in rat myocytes during beta-adrenergic stimulation. J Physiol 505(Pt 2):385–402. doi:10.1111/j.1469-7793.1997.385bb.x
Kettlewell S, Burton FL, Smith GL, Workman AJ (2013) Chronic myocardial infarction promotes atrial action potential alternans, afterdepolarizations, and fibrillation. Cardiovasc Res 99:215–224. doi:10.1093/cvr/cvt087
Kockskamper J, Blatter LA (2002) Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol 545:65–79. doi:10.1111/j.1469-7793.1997.385bb.x
Kodama M, Kato K, Hirono S, Okura Y, Hanawa H, Ito M, Fuse K, Shiono T, Watanabe K, Aizawa Y (2001) Mechanical alternans in patients with chronic heart failure. J Card Fail 7:138–145. doi:10.1054/jcaf.2001.24122
Komiya N, Seto S, Nakao K, Yano K (2005) The influence of beta-adrenergic agonists and antagonists on T-wave alternans in patients with and without ventricular tachyarrhythmia. Pacing Clin Electrophysiol 28:680–684. doi:10.1111/j.1540-8159.2005.00146.x
Lenski M, Schleider G, Kohlhaas M, Adrian L, Adam O, Tian Q, Kaestner L, Lipp P, Lehrke M, Maack C, Bohm M, Laufs U (2015) Arrhythmia causes lipid accumulation and reduced glucose uptake. Basic Res Cardiol 110:40. doi:10.1007/s00395-015-0497-2
Llach A, Molina CE, Fernandes J, Padro J, Cinca J, Hove-Madsen L (2011) Sarcoplasmic reticulum and l-type Ca2+ channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J Physiol. doi:10.1113/jphysiol.2010.197715
Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casado V, Ciruela F, Lluis C, Franco R, Cinca J, Hove-Madsen L (2011) Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J 32:721–729. doi:10.1093/eurheartj/ehq464
Lugo CA, Cantalapiedra IR, Penaranda A, Hove-Madsen L, Echebarria B (2014) Are SR Ca content fluctuations or SR refractoriness the key to atrial cardiac alternans?: Insights from a human atrial model. Am J Physiol Heart Circ Physiol 306:H1540–H1552. doi:10.1152/ajpheart.00515.2013
Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD (2004) The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci 117:6327–6337. doi:10.1242/jcs.01559
Narayan SM, Bayer JD, Lalani G, Trayanova NA (2008) Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol 52:1782–1792. doi:10.1016/j.jacc.2008.08.037
Narayan SM, Bode F, Karasik PL, Franz MR (2002) Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation 106:1968–1973. doi:10.1161/01.CIR.0000037062.35762.B4
Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS (2010) CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 106:1134–1144. doi:10.1161/CIRCRESAHA.109.203836
Olah ME (1997) Identification of A2a adenosine receptor domains involved in selective coupling to Gs. Analysis of chimeric A1/A2a adenosine receptors. J Biol Chem 272:337–344. doi:10.1074/jbc.272.1.337
Olah ME, Stiles GL (1992) Adenosine receptors. Annu Rev Physiol 54:211–225. doi:10.1146/annurev.ph.54.030192.001235
Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS (1999) Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99:1385–1394. doi:10.1161/01.CIR.99.10.1385
Picht E, DeSantiago J, Blatter LA, Bers DM (2006) Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res 99:740–748. doi:10.1161/01.RES.0000244002.88813.91
Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ (1994) Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 330:235–241. doi:10.1056/NEJM199401273300402
Shusterman V, Goldberg A, London B (2006) Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation 113:2880–2887. doi:10.1161/CIRCULATIONAHA.105.607895
Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM (1999) Atrial l-type Ca2+ currents and human atrial fibrillation. Circ Res 85:428–436. doi:10.1161/01.RES.85.5.428
Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM (1997) Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res 80:772–781
Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR (2005) Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111:2025–2032. doi:10.1161/01.CIR.0000162461.67140.4C
Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D (2014) Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 129:145–156. doi:10.1161/CIRCULATIONAHA.113.006641
Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D (2012) Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+–Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125:2059–2070. doi:10.1161/CIRCULATIONAHA.111.067306
Workman AJ, Kane KA, Rankin AC (1999) Ionic basis of a differential effect of adenosine on refractoriness in rabbit AV nodal and atrial isolated myocytes. Cardiovasc Res 43:974–984 (S0008-6363(99)00166-2 [pii])
Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR (2007) Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J Biol Chem 282:30256–30264. doi:10.1074/jbc.M703510200
Acknowledgments
We would like to thank Mr. Andreu Ferrero-Gregori for invaluable help with the statistical analysis and greatly appreciate the collaboration of the surgeons at the Cardiac Surgery Department at Hospital de la Santa Creu i Sant Pau.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Funding
This work has received funding from Spanish Ministry of Economía y Competitividad, Instituto de Salud Carlos III and Fondo Europeo de Desarollo Regional (FEDER): (SAF2011-30312), (SAF2014-58286-C2-1R), (CNIC2009-08) and (network RD12/0042/0002), Generalitat de Catalunya (2014SGR1465) and Marie Curie IEF Grant (PIEF-GA-2012-331241).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Molina, C.E., Llach, A., Herraiz-Martínez, A. et al. Prevention of adenosine A2A receptor activation diminishes beat-to-beat alternation in human atrial myocytes. Basic Res Cardiol 111, 5 (2016). https://doi.org/10.1007/s00395-015-0525-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0525-2