Abstract
Purpose
Inflammatory bowel diseases (IBD) are characterized by severe inflammation within the gastrointestinal (GI) tract. This inflammation is known to drive the catabolism of protein in the affected tissue and modulate systemic protein metabolism. Yet despite the established increase in oxidative stress and changes in protein catabolism, little is known as to the effects of IBD on metabolism of glutathione (GSH) and related metabolites. The aim of this study was to conduct a comprehensive analysis of the response of GSH and related sulfhydryl metabolites to malnutrition and GI inflammation. We hypothesized that the inflammatory stress of colitis would decrease the concentration and the synthesis of GSH in various tissues of well-nourished piglets. Additionally, the superimposition of malnutrition on colitis would further decrease glutathione status.
Methods
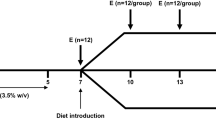
Healthy, well-nourished piglets were compared to those receiving dextran sulphate sodium-induced, a macronutrient-restricted diet or both. The synthesis of GSH was determined by primed constant infusion of [15N,13C2]glycine and tandem mass spectrometry analysis. Additionally, the concentrations of GSH and related sulfhydryl metabolites were also determined by UHPLC–tandem mass spectrometry—a novel analytic technique.
Results
In healthy piglets, GSH synthesis was highest in the liver, along with the concentrations of both cysteine and γ-glutamylcysteine. Piglets with colitis had decreased synthesis of GSH and decreased concentrations of GSH, cysteine and γ-glutamylcysteine in the distal colon compared to healthy controls. Additionally, there was no change with superimposition of malnutrition on colitis in the distal colon.
Conclusion
Synthesis and metabolism of GSH are uniquely regulated in each tissue. Colitis, independent of nutrition, compromises GSH status and the concentration of cysteine in the distal colon of piglets with GI inflammation. The techniques developed in this study have translational applications and can be scaled for use in clinical investigation of GI inflammation.



Similar content being viewed by others
References
Griffiths A, Nguyen P, Smith C, MacMillan J, Sherman P (1993) Growth and clinical course of children with Crohn’s disease. Gut 34:939–943
Markowitz J, Daum F (1994) Growth impairment in pediatric inflammatory bowel disease. Am J Gastroenterol 89:319–326
Hartman C, Eliakim R, Shamir R (2009) Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol 15:2570–2578
Sawczenko A, Sandhu B (2003) Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child 88:995–1000
Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Droge W (1998) Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut 42:485–492
Sokol R, Hoffenberg E (1996) Antioxidants in pediatric gastrointestinal disease. Pediatr Clin N Am 43:471–488
O’Sullivan M, O’Morain C (2006) Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol 20:561–573
Lichtenstein G, Abreu M, Cohen R, Tremaine W (2006) American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 130:935–939
Dignass A, Lindsay J, Sturm A, Windsor A, Colombel J, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G, Oresland T, Reinisch W, Sans M, Stange E, Vermeire S, Travis S, Van Assche G (2012) Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 6:991–1030
Canalis E, Bilezikian J, Angell A, Glustina A (2004) Perspectives on glucocorticoid-induced osteoporosis. Bone 34:593–598
Lei X (2002) In vivo antioxidant role of glutathione peroxidase: evidence from knockout mice. Methods Enzymol 347:213–225
Fang Y-Z, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Huseby N-E, Sundkvist E, Svineng G (2009) Glutathione and sulfur containing amino acids: antioxidant and conjugation activities. In: Mazza G, Masella R (eds) Glutathione and sulfur amino acids in human health and disease. Wiley, New Jersey
Meister A (1974) Glutathione; Metabolism and function via the γ-glutamyl cycle. Life Sci 15:177–190
Orlowski M, Meister A (1970) The γ-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci 67:1248–1255
Kozak E, Tate S (1982) Glutathione-degrading enzymes of microvillus membranes. J Biol Chem 257:6322–6327
Cappiello M, Lazzarotti A, Buono F, Scaloni A, D’Ambrosio C, Amodeo P, Mendez B, Pelosi P, Del Corso A, Mura U (2004) New role for leucyl aminopeptidase in glutathione turnover. Biochem J 378:35–44
Iantomasi T, Marraccini P, Favilli F, Vincenzini M, Ferretti P, Tonelli F (1994) Glutathione metabolism in Crohn’s disease. Biochem Med Met Biol 53:87–91
Karp S, Koch T (2006) Oxidative stress and antioxidants in inflammatory bowel disease. Dis Mon 52:199–207
Holmes E, Yong S, Eiznhamer D, Kesavarzian A (1998) Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci 43:1088–1095
Sturniolo G, Mestriner C, Lecis P, D’Odorico A, Venturi C, Irato P, Cecchetto A, Tropea A, Longo G, D’Inca R (1998) Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scand J Gastroentrol 33:644–649
Jahoor F, Wykes L, Reeds P, Henry J, Del Rosario M, Frazer E (1995) Protein-deficient pigs cannot maintain reduced glutathione homeostasis when subjected to the stress of inflammation. J Nutr 125:1462–1472
Reid M, Badaloo A, Forrester T, Morlese J, Frazer M, Heird W, Jahoor F (2000) In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab 278:E405–E412
Wykes L, Ball R, Pencharz P (1993) Development and validation of a total parenteral nutritional model in the neonatal piglet. J Nutr 123:1248–1259
Benight N, Burrin D, Barbara S (2009) Intestinal metabolism of sulfur amino acids. In: Mazza G, Masella R (eds) Glutathione and sulfur amino acids in human health and disease. Wiley, New Jersey
Miller E, Ullrey D (1987) The pig as a model for human nutrition. Annu Rev Nutr 7:361–382
Harding SV, Adegoke OA, Fraser KG, Marliss EB, Chevalier S, Kimball SR, Jefferson LS, Wykes LJ (2010) Maintaining adequate nutrition, not probiotic administration, prevents growth stunting and maintains skeletal muscle protein synthesis rates in a piglet model of colitis. Pediatr Res 67(3):268–273
Harding S, Fraser K, Wykes L (2008) Probiotics stimulate liver and plasma protein synthesis in piglets with dextran sulfate-induced colitis and macronutrient restriction. J Nutr 138:2129–2135
NRC (1998) Nutrient requirements of swine, 10th rev. National Academy Press, Washington
Canadian Councilon Animal Care (1993) Guide to the care and use of experimental animals. Bradda Printing Services Inc., Ottawa
Kim C, Kovacs-Nolan J, Yang C, Archbold T, Fan M, Mine Y (2009) L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim Biophys Acta 1790:1161–1169
Clapper M, Adrian R, Pfeiffer G, Kido K, Everley L, Cooper H, Murthy S (1999) Depletion of colonic detoxication enzyme activity in mice with dextran sulphate sodium-induced colitis. Aliment Pharmacol Ther 13:389–396
Edalat M, Mannervik B, Axelsson L (2004) Selective expression of detoxifying glutathione transferases in mouse colon: effect of experimental colitis and the presence of bacteria. Histochem Cell Biol 122:151–159
Bhaskar L, Ramakrishna B, Balasubramanian K (1995) Colonic mucosal antioxidant enzymes and lipid peroxide levels in normal subjects and patients with ulcerative colitis. J Gastroenterol Hepatol 10:140–143
Kaplowitz N, Aw T, Ookhtens M (1985) The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 25:715–744
Meredith M, Reed D (1982) Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem 257:3747–3753
Griffith O (1999) Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27:922–935
Ookhtens M, Kaplowitz N (1998) Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis 18:313–329
Badaloo A, Reid M, Forrester T, Heird W, Jahoor F (2002) Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr 76:646–652
Badaloo A, Hsu J, Taylor-Bryan C, Green C, Reid M, Forrester T, Jahoor F (2012) Dietary cysteine is used more efficiently by children with severe acute malnutrition with edema compared with those without edema. Am J Clin Nutr 95:84–90
Beutler E (1975) Red cell metabolism: a manual of biochemical methods, 2nd edn. Grune & Sratton, New York
Richie JJ, Abraham P, Leutzinger Y (1996) Long-term stability of blood glutathione and cysteine in humans. Clin Chem 42:1100–1105
Richie JJ, Skowronski L, Abraham P, Leutzinger Y (1996) Blood glutathione concentrations in a large-scale human study. Clin Chem 42:64–70
Miralles-Barrachina O, Savoye G, Belmonte-Zalar L, Hochain P, Ducrotte P, Hecketsweiller B, Lerebours E, Dechelotte P (1999) Low levels of glutathione in endoscopic biopsies of patients with Crohn’s colitis: the role of malnutrition. Clin Nutr 18:313–317
Sen C (2000) Cellular thiols and redox-regulated signal transduction. Curr Top Cell Regul 36:1–30
DeLeve L, Kaplowitz N (1991) Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther 52:287–305
Meister A (1988) Glutathione. In: Aria I, Jakoby W, Popper H, Schachter D, Shafritz D (eds) The liver: biology and phathobiobiology, 2nd edn. Raven Press, New York, pp 401–417
Poon J, Josephy D (2012) Hydrolysis of S-aryl-cysteinylglycine conjugates catalyzed by porcine kidney cortex membrane dipeptidase. Xenobiotica 42:1178–1186
Griffith O, Meister A (1979) Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci 76:5606–5610
Anderson M, Bridges R, Meister A (1980) Direct evidence for inter-organ transport of glutathione utilization involves γ-glutamyl transpeptidase. Biochem Biophys Res Commun 96:848–853
Bartoli G, Haberle D, Sies H (1978) Glutathione efflux from perfused rat liver and its relation to glutathione uptake by the kidney. Springer, New York
Acknowledgments
Funding
Partially funded by: (1) Natural Sciences and Engineering Research Council of Canada Grant Number 183710-09 (Wykes), (2) Canadian Foundation for Innovation Grant Number 23910 (Wykes) and (3) McGill University Graduate Excellence Fellowship (Vassilyadi).
Author contributions
All authors read and approved the final manuscript. Vassilyadi, Harding, Nitschmann and Wykes designed the research; Harding conducted the animal trial and isotope infusions; Vassilyadi and Nitschmann performed mass spectrometry; Vassilyadi performed statistical analysis; Vassilyadi, Harding, Nitschmann and Wykes wrote the paper; and Wykes had primary responsibility for the final content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vassilyadi, P., Harding, S.V., Nitschmann, E. et al. Experimental colitis and malnutrition differentially affect the metabolism of glutathione and related sulfhydryl metabolites in different tissues. Eur J Nutr 55, 1769–1776 (2016). https://doi.org/10.1007/s00394-015-0995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0995-x