Abstract
Purpose
The liver in biliary atresia (BA) is characterized by progressing fibrosis which is promoted by unclear reasons. We aimed to understand the factors influencing liver fibrosis. This study hypothesized that HPCs (hepatic progenitor cells) are activated and associated with liver fibrosis in biliary atresia.
Methods
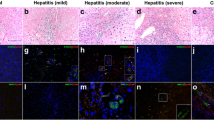
Liver samples from biliary atresia patients are as BA group, and the normal liver derived from hepatoblastoma infants during operation are control group. The extent of fibrosis in liver samples was blindly evaluated by two experienced pathologists depending on Ishak system. The BA liver samples were divided into mild liver fibrosis group (grade I–IV, BAa) and severe liver fibrosis group (grade V–VI, BAb) to detect Fn14 protein expression.
Results
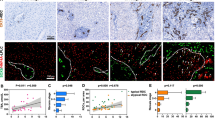
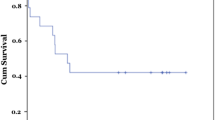
In mRNA level, Fn14 expression was 21.23 ± 8.3 vs. 1.00 ± 0.17, p = 0.023 < 0.05 and CD133 expression was 6.02 ± 2.16 vs. 1.14 ± 0.75, p = 0.008 < 0.01 between BA group and control group. Fn14 cells co-expressed the progenitor marker CD133 in liver, and activated in BA. Fn14 andα-SMA were co-location in fibrous area in liver. Compared to the control group, Fn14, CD133, and α-SMA protein expression were 2.10 ± 0.53 vs. 0.97 ± 0.2, p = 0.001, 2.23 ± 0.57 vs. 1.00 ± 0.03, p = 0.000, 4.96 ± 2.4 vs. 1.00 ± 0.22, p = 0.001. The Fn14 protein expression was 2.60 ± 0.35 vs. 1.86 ± 0.42, p = 0.012, between BAb and BAa group.
Conclusion
Fn14 cells, which co-express the progenitor marker CD133 in liver, are HPCs and activated in BA. Fn14 + HPCs are associated with liver fibrosis in BA.




Similar content being viewed by others
References
McKiernan PJ, Baker AJ, Kelly DA (2000) The frequency and outcome of biliary atresia in the UK and Ireland. Lancet 355:25–29. doi:10.1016/s0140-6736(99)03492-3
de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ (2011) Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 9:1086–1091. doi:10.1016/j.cgh.2011.07.024
Karrer FM, Price MR, Bensard DD, Sokol RJ, Narkewicz MR, Smith DJ, Lilly JR (1996) Long-term results with the Kasai operation for biliary atresia. Arch Surg 131:493–496
Carceller A, Blanchard H, Alvarez F, St-Vil D, Bensoussan AL, Di Lorenzo M (2000) Past and future of biliary atresia. J Pediatr Surg 35:717–720. doi:10.1053/jpsu.2000.6034
Pakarinen MP, Rintala RJ (2011) Surgery of biliary atresia. Scand J Surg 100:49–53
Mack CL, Feldman AG, Sokol RJ (2012) Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis 32:307–316. doi:10.1055/s-0032-1329899
Paku S, Schnur J, Nagy P, Thorgeirsson SS (2001) Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol 158:1313–1323. doi:10.1016/s0002-9440(10)64082-5
Petersen BE, Zajac VF, Michalopoulos GK (1998) Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology 27:1030–1038. doi:10.1002/hep.510270419
Sell S (2001) Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 33:738–750. doi:10.1053/jhep.2001.21900
Newsome PN, Hussain MA, Theise ND (2004) Hepatic oval cells: helping redefine a paradigm in stem cell biology. Curr Top Dev Biol 61:1–28. doi:10.1016/s0070-2153(04)61001-5
Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ, Forbes SJ (2015) Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol 17:971–983. doi:10.1038/ncb3203
Lorenzini S, Bird TG, Boulter L, Bellamy C, Samuel K, Aucott R, Clayton E, Andreone P, Bernardi M, Golding M, Alison MR, Iredale JP, Forbes SJ (2010) Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 59:645–654. doi:10.1136/gut.2009.182345
Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, Thomas JA, Wojtacha D, Gambardella A, Sansom OJ, Iredale JP, Forbes SJ (2013) Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA 110:6542–6547. doi:10.1073/pnas.1302168110
Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, Knight B (2010) Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 52:291–302. doi:10.1002/hep.23663
Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang B, Bissell DM, Burkly LC (2005) TWEAK induces liver progenitor cell proliferation. J Clin Invest 115:2330–2340. doi:10.1172/jci23486
Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA (1999) The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 274:33166–33176
Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, Peifley KA, Winkles JA (2000) The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 156:1253–1261. doi:10.1016/s0002-9440(10)64996-6
Ho DH, Vu H, Brown SA, Donohue PJ, Hanscom HN, Winkles JA (2004) Soluble tumor necrosis factor-like weak inducer of apoptosis overexpression in HEK293 cells promotes tumor growth and angiogenesis in athymic nude mice. Cancer Res 64:8968–8972. doi:10.1158/0008-5472.can-04-1879
Kawakita T, Shiraki K, Yamanaka Y, Yamaguchi Y, Saitou Y, Enokimura N, Yamamoto N, Okano H, Sugimoto K, Murata K, Nakano T (2004) Functional expression of TWEAK in human hepatocellular carcinoma: possible implication in cell proliferation and tumor angiogenesis. Biochem Biophys Res Commun 318:726–733. doi:10.1016/j.bbrc.2004.04.084
Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL (1997) TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272:32401–32410
Brown SA, Ghosh A, Winkles JA (2010) Full-length, membrane-anchored TWEAK can function as a juxtacrine signaling molecule and activate the NF-kappaB pathway. J Biol Chem 285:17432–17441. doi:10.1074/jbc.M110.131979
Karaca G, Swiderska-Syn M, Xie G, Syn WK, Kruger L, Machado MV, Garman K, Choi SS, Michelotti GA, Burkly LC, Ochoa B, Diehl AM (2014) TWEAK/Fn14 signaling is required for liver regeneration after partial hepatectomy in mice. PLoS One 9:e83987. doi:10.1371/journal.pone.0083987
Affo S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millan C, Loaeza-del-Castillo A, Altamirano J, Garcia-Pagan JC, Arroyo V, Gines P, Caballeria J, Schwabe RF, Bataller R (2013) Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62:452–460. doi:10.1136/gutjnl-2011-301146
Mavila N, James D, Shivakumar P, Nguyen MV, Utley S, Mak K, Wu A, Zhou S, Wang L, Vendyres C, Groff M, Asahina K, Wang KS (2014) Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 60:941–953. doi:10.1002/hep.27203
Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, Hanto DW, Otterbein LE, Popov Y (2013) Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 183:182–194. doi:10.1016/j.ajpath.2013.03.018
Friedman SL (2004) Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1:98–105. doi:10.1038/ncpgasthep0055
Ruddell RG, Knight B, Tirnitz-Parker JE, Akhurst B, Summerville L, Subramaniam VN, Olynyk JK, Ramm GA (2009) Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology 49:227–239. doi:10.1002/hep.22597
Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR (2005) Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 41:809–818. doi:10.1002/hep.20650
Knight B, Akhurst B, Matthews VB, Ruddell RG, Ramm GA, Abraham LJ, Olynyk JK, Yeoh GC (2007) Attenuated liver progenitor (oval) cell and fibrogenic responses to the choline deficient, ethionine supplemented diet in the BALB/c inbred strain of mice. J Hepatol 46:134–141. doi:10.1016/j.jhep.2006.08.015
Knight B, Lim R, Yeoh GC, Olynyk JK (2007) Interferon-gamma exacerbates liver damage, the hepatic progenitor cell response and fibrosis in a mouse model of chronic liver injury. J Hepatol 47:826–833. doi:10.1016/j.jhep.2007.06.022
Dwyer BJ, Olynyk JK, Ramm GA, Tirnitz-Parker JE (2014) TWEAK and LTbeta signaling during chronic liver disease. Front Immunol 5:39. doi:10.3389/fimmu.2014.00039
Acknowledgements
This study was supported by the frontier technology research project of Shanghai Shenkang Hospital Development Center (SHDC12013124).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded the frontier technology research project of Shanghai Shenkang Hospital Development Center (grant number SHDC12013124).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zheng, L., Lv, Z., Gong, Z. et al. Fn14 hepatic progenitor cells are associated with liver fibrosis in biliary atresia. Pediatr Surg Int 33, 593–599 (2017). https://doi.org/10.1007/s00383-017-4068-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-017-4068-5