Abstract
Introduction
Oesophageal atresia/tracheo-oesophageal atresia (OA/TOF) frequently arises with associated anomalies and has been clinically linked with 22q11 deletion syndromes, a group of conditions due to Tbx1 gene mutation which include Di George syndrome. Tbx1 and Tbx2 genes modulate pharyngeal and cardiac development, but are also expressed in the developing foregut and are known to interact with key signalling pathways described in oesophageal formation including bone morphogenic proteins. The adriamycin mouse model (AMM) reliably displays OA/TOF-like foregut malformations providing a powerful system for investigating the disturbances in gene regulation and morphology involved in tracheo-oesophageal malformations. We hypothesised that foregut abnormalities observed in the AMM are associated with altered Tbx1 and Tbx2 gene expression.
Methods
Time-mated CBA/Ca mice received intra-peritoneal injection of adriamycin (for treated) or saline (for controls) on embryonic days (E)7 and 8. Untreated Cd1 embryos were used to establish normal expression patterns. Embryos harvested on E9–E11 underwent whole-mount in situ hybridization with labelled RNA probes for Tbx1 and Tbx2. Optical projection tomography was used to visualise expression in whole embryos by 3D imaging.
Results
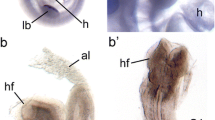
Tbx1 expression was visualised in a highly specific pattern in the proximal oesophageal endoderm in normal and control embryos. In the AMM, extensive ectopic expression of Tbx1 was detected in the dorsal foregut and adjacent to the TOF. The focally restricted oesophageal expression pattern persisted in the AMM, but was posteriorly displaced in relation to the tracheal bifurcation. Tbx2 was widely expressed in the ventral foregut mesoderm of controls, lacking specific endoderm localisation. In the AMM, altered Tbx2 expression in the foregut was only seen in severely affected embryos.
Conclusion
Highly specific Tbx1 expression in the proximal oesophageal endoderm suggests that Tbx1 may be an important regulator of normal oesophageal development. Altered Tbx1 expression in dorsal foregut and adjacent to the TOF in the AMM suggests that Tbx1 gene disruption may contribute to the pathogenesis of tracheo-oesophageal malformations.



Similar content being viewed by others
References
Gray SW, Skandalakis JE (1972) Embryology for Surgeons. W.B. Saunders, Philadelphia, pp 63–100
Grapin-Botton A, Constam D (2007) Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev 124(4):253–278
Pedersen RN, Calzolari E, Husby S, Garne E (2012) Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child 97(3):227–232
Solomon BD (2011) VACTERL/VATER association. Orphanet J Rare Dis 6:56
Shaw-Smith C (2006) Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet 43(7):545–554
Mc Laughlin D, Hajduk P, Murphy P, Puri P (2013) Adriamycin-induced models of VACTERL association. Mol Syndromol 4(1–2):46–62
Hajduk P, Sato H, Puri P, Murphy P (2011) Abnormal notochord branching is associated with foregut malformations in the adriamycin treated mouse model. PLoS ONE 6(11):e27635
Papaioannou VE, Silver LM (1998) The T-box gene family. BioEssays 20(1):9–19
van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8(1):50–60
Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R (2001) TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 104(4):619–629
Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, Brown MC, Adams J, Morrow BE (2004) Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet 13(15):1577–1585
Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A (2001) Tbx1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410(6824):97–101
Mesbah K, Rana, Francou A, van duijvenboden K, Papaioannou VE, Moorman AF, Kelly RG, Christoffels VM (2012) Identification of a Tbx1/Tbx2/Tbx3 genetic pathway governing pharyngeal and arterial pole morphogenesis. Hum Mol Genet 21(6):1217–1229
Jerome LA, Papaioannou VE (2001) DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet 27(3):286–291
Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE (1996) Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn 206(4):379–390
Lee KD, Okazaki T, Kato Y, Lane GJ, Yamataka A (2008) Esophageal atresia and tracheo-esophageal fistula associated with coarctation of the aorta, CHARGE association, and DiGeorge syndrome: a case report and literature review. Pediatr Surg Int 24(10):1153–1156
Bergman JE, Janssen N, Hoefsloot LH, Jongmans MC, Hofstra RM, van Ravenswaaij-Arts CM (2011) CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J Med Genet 48(5):334–342
Corsten-Janssen N, Saitta SC, Hoefsloot LH, McDonald-Mcginn DM, Driscoll DA, Derks R, Dickinson KA, Kerstjens-Frederikse WS, Emanuel BS, Zackai EH, Van Ravenswaaij-Arts CMA (2013) More clinical overlap between 22q11.2 deletion syndrome and charge syndrome than often anticipated. Mol Syndromol 4(5):235–245
Iafolla AK, McConkie-Rosell A, Chen YT (1991) VATER and hydrocephalus: distinct syndrome? Am J Med Genet 38(1):46–51
Meins M, Burfeind P, Motsch S, Trappe R, Bartmus D, Langer S, Speicher MR, Muhlendyck H, Bartels I, Zoll B (2003) Partial trisomy of chromosome 22 resulting from an interstitial duplication of 22q11.2 in a child with typical cat eye syndrome. J Med Genet 40(5):e62
Schramm C, Draaken M, Bartels E, Boemers TM, Aretz S, Brockschmidt FF, Nothen MM, Ludwig M, Reutter H (2011) De novo microduplication at 22q11.21 in a patient with VACTERL association. Eur J Med Genet 54(1):9–13
Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE (1996) Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn 206(4):379–390
Sakiyama J, Yamagishi A, Kuroiwa A (2003) Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development 130(7):1225–1234
Hajduk P, Murphy P, Puri P (2010) Mesenchymal expression of Tbx4 gene is not altered in adriamycin mouse model. Pediatr Surg Int 26(4):407–411
Sharpe J, Ahlgren U, Perry P, Hill B, Ross A, Hecksher-Sorensen J, Baldock R, Davidson D (2002) Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296(5567):541–545
Theiler K (1989) The house mouse: atlas of embryonic development. Springer, Berlin
Summerhurst K, Stark M, Sharpe J, Davidson D, Murphy P (2008) 3D representation of Wnt and Frizzled gene expression patterns in the mouse embryo at embryonic day 11.5 (Ts19). Gene Expr Patterns 8(5):331–348
Felix JF, de Jong EM, Torfs CP, de Klein A, Rottier RJ, Tibboel D (2009) Genetic and environmental factors in the etiology of esophageal atresia and/or tracheoesophageal fistula: an overview of the current concepts. Birth Defects Res A Clin Mol Teratol 85(9):747–754
Liu M, Wu X, Xu J, Jin R (2009) Influence of retinoic acid on TBX1 expression in myocardial cells induced by Shh and Fgf8. Front Med China 3(1):61–66
Zhang L, Zhong T, Wang Y, Jiang Q, Song H, Gui Y (2006) TBX1, a DiGeorge syndrome candidate gene, is inhibited by retinoic acid. Int J Dev Biol 50(1):55–61
Bachiller D, Klingensmith J, Shneyder N, Tran U, Anderson R, Rossant J, De Robertis EM (2003) The role of chordin/Bmp signals mammalian pharyngeal development and DiGeorge syndrome. Development 130(15):3567–3578
Fong SH, Emelyanov A, Teh C, Korzh V (2005) Wnt signalling mediated by Tbx2b regulates cell migration during formation of the neural plate. Development 132(16):3587–3596
Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M (2006) Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev Cell 11(6):873–885
Chen L, Fulcoli FG, Tang S, Baldini A (2009) Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res 105(9):842–851
Williams AK, Quan QB, Beasley SW (2003) Three-dimensional imaging clarifies the process of tracheoesophageal separation in the rat. J Pediatr Surg 38(2):173–177
Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A (2002) Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet 11(8):915–922
Moraes F, Novoa A, Jerome-Majewska LA, Papaioannou VE, Mallo M (2005) Tbx1 is required for proper neural crest migration and to stabilize spatial patterns during middle and inner ear development. Mech Dev 122(2):199–212
Calmont A, Thapar N, Scambler PJ, Burns AJ (2011) Absence of the vagus nerve in the stomach of Tbx1-/- mutant mice. Neurogastroenterol Motil 23(2):125–130
Zweier C, Sticht H, Aydin-Yaylag Ã, Campbell CE, Rauch A (2007) Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am J Hum Genet 80(3):510–517
Freyer L, Nowotschin S, Pirity MK, Baldini A, Morrow BE (2013) Conditional and constitutive expression of a Tbx1-GFP fusion protein in mice. BMC Dev Biol 13(1):33
Abu-Issa R, Smyth G, Smoak I, Yamamura KI, Meyers EN (2002) Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129(19):4613–4625
Urness LD, Bleyl SB, Wright TJ, Moon AM, Mansour SL (1996) Redundant and dosage sensitive requirements for Fgf3 and Fgf10 in cardiovascular development. Dev Biol 356(2):383–397
Behesti H, Holt JKL, Sowden JC (2006) The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol 15(6):62
Litingtung Y, Lei L, Westphal H, Chiang C (1998) Sonic hedgehog is essential to foregut development. Nat Genet 20(1):58–61
Fulcoli FG, Huynh T, Scambler PJ, Baldini A (2009) Tbx1 regulates the BMP-smad1 pathway in a transcription independent manner. PLoS ONE 4(6):e6049
Nie X, Brown CB, Wang Q, Jiao K (2011) Inactivation of Bmp4 from the Tbx1 expression domain causes abnormal pharyngeal arch artery and cardiac outflow tract remodeling. Cells Tissues Organs 193(6):393–403
Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL (2006) Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 74(7):422–437
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mc Laughlin, D., Murphy, P. & Puri, P. Altered Tbx1 gene expression is associated with abnormal oesophageal development in the adriamycin mouse model of oesophageal atresia/tracheo-oesophageal fistula. Pediatr Surg Int 30, 143–149 (2014). https://doi.org/10.1007/s00383-013-3455-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-013-3455-9