Abstract
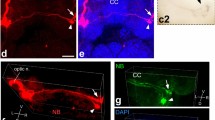
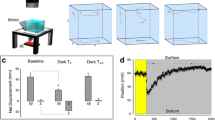
In the terrestrial slug, Limax, eyes are located at the tip of the superior tentacles. This animal has long been believed to show negative phototaxis through tropotaxis, i.e., it compares the two light intensities detected by bilateral eyes to move away from a light source. As one of the possible manifestations of such negative phototaxis, a circling movement has been observed: if one of the superior tentacles is removed, the slugs continuously move in the direction of the removed side. However, there has been no evidence demonstrating that this behavior is actually based on negative phototropotaxis. In this study, we showed that the slugs do not exhibit the circling behavior in the absence of light, and that amputation of the cerebral commissure also diminishes the circling behavior under light. We could detect light-evoked responses during electrical recording from the cut edge of the cerebral commissure. Labeling of the optic nerve with neurobiotin also revealed the presence of the commissural fibers that potentially transmit the light information to the contralateral cerebral ganglion. Our study suggests that the slug’s circling behavior is based on phototropotaxis in which the light intensities detected by the bilateral eyes are compared through the cerebral commissure.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BSA:
-
Bovine serum albumin
- CC:
-
Cerebral commissure
- CCD:
-
Charge-coupled device
- ChAT:
-
Cholineacetyltransferase
- DAPI:
-
4′,6-diamino-2-phenylindole
- FMRFamide:
-
Phe-Met-Arg-Phe-NH2
- GABA:
-
γ-aminobutyric acid
- 5-HT:
-
5-hydroxytryptamine (serotonin)
- PBS:
-
Phosphate-buffered saline
- ST:
-
Superior tentacle
References
Chase R, Croll RP (1981) Tentacular function in snail olfactory orientation. J Comp Physiol 143:357–362
Chono K, Fujito Y, Ito E (2002) Non-ocular dermal photoreception in the pond snail Lymnaea stagnalis. Brain Res 951:107–112
Croll RP (1983) Gastropod chemoreception. Biol Rev 58:293–319
Crow TJ, Alkon DL (1980) Associative behavioral modification in Hermissenda: cellular correlates. Science 209:412–414
Crozier WJ, Federighi H (1924a) Phototropic circus movements of Limax affected by temperature. J Gen Physiol 7:151–169
Crozier WJ, Federighi H (1924b) Suppression of phototropic circus movements of Limax by strychnine. J Gen Physiol 7:221–224
Farley J, Alkon DL (1982) Associative neural and behavioral changes in Hermissenda: consequences of nervous system orientation for light and pairing specificity. J Neurophysiol 48:785–807
Friedrich A, Teyke T (1998) Identification of stimuli and input pathways mediating food-attraction conditioning in the snail, Helix. J Comp Physiol A 183:247–254
Gomez-Marin A, Louis M (2012) Active sensation during orientation behavior in the Drosophila larva: more sense than luck. Curr Opin Neurobiol 22:208–215
Gotow T (1975) Morphology and function of the photoexcitable neurones in the central ganglia of Onchidium verruculatum. J Comp Physiol 99:139–152
Gotow T, Tateda H, Kuwabara M (1973) The function of photoexcitable neurons in the central ganglia for behavioral activity of the marine mollusc, Onchidium verruculatum. J Comp Physiol 83:361–376
Heldman E, Grossman Y, Jerussi TP, Alkon DL (1979) Cholinergic features of photocereptor synapses in Hermissenda. J Neurophysiol 42:153–165
Hisano N, Terada H, Kuwabara M (1972) Photosensitive neurones in the marine pulmonate mollusc Onchidium verruculatum. J Exp Biol 57:651–660
Jékely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nédélec F, Arendt D (2008) Mechanism of phototaxis in marine zooplankton. Nature 456:395–400
Kartelija G, Nedeljkovic N, Radenovic L (2003) Photosensitive neurons in mollusks. Comp Biochem Physiol A 134:483–495
Kobayashi S, Hattori M, Elekes K, Ito E, Matsuo R (2010) FMRFamide regulates oscillatory activity of the olfactory center in a slug. Eur J Neurosci 32:1180–1192
Kobayashi S, Matsuo R, Sadamoto H, Watanabe S, Ito E (2012) Excitatory effects of GABA on procerebrum neurons in a slug. J Neurophysiol 108:989–998
Lederhendler II, Alkon DL (1987) Associatively reduced withdrawal from shadows in Hermissenda: a direct behavioral analog of photoreceptor to brief light steps. Behav Neural Biol 47:227–249
Matsuo R, Kawaguchi E, Yamagishi M, Amano T, Ito E (2010) Unilateral memory storage in the procerebrum of the terrestrial slug Limax. Neurobiol Learn Mem 93:337–342
Matsuo R, Kobayashi S, Yamagishi M, Ito E (2011) Two pairs of tentacles and a pair of procerebra: optimized functions and redundant structures in the sensory and central organs involved in olfactory learning of terrestrial pulmonates. J Exp Biol 214:879–886
Matsuo R, Yamagishi M, Wakiya K, Tanaka Y, Ito E (2013) Target innervation is necessary for neuronal polyploidization in the terrestrial slug Limax. Dev Neurobiol 73:609–620
Matsuo R, Kobayashi S, Wakiya K, Yamagishi M, Fukuoka M, Ito E (2014) The cholinergic system in the olfactory center of the terrestrial slug Limax. J Comp Neurol 522:2951–2966
Michel S, Schoch K, Stevenson PA (2000) Amine and amino acid transmitters in the eye of the mollusc Bulla gouldiana: an immunocytochemical study. J Comp Neurol 425:244–256
Olson LM, Jacklet JW (1985) The circadian pacemaker in the Aplysia eye sends axons throughout the central nervous system. J Neurosci 5:3214–3227
Pankey S, Sunada H, Horikoshi T, Sakakibara M (2010) Cyclic nucleotide-gated channels are involved in phototransduction of dermal photoreceptors in Lymnaea stagnalis. J Comp Physiol B 180:1205–1211
Pašić M, Kartelija G (1990) Modification of the action potential of Helix pamatia photosensitive neurons by light. Comp Biochem Physiol 95A:73–78
Sunada H, Sakaguchi T, Horikoshi T, Lukowiak K, Sakakibara M (2010) The shadow-induced withdrawal response, dermal photoreceptors, and their input to the higher-order interneuron RPeD11 in the pond snail Lymnaea stagnalis. J Exp Biol 213:3409–3415
Tuchina OP, Zhukov VV, Meyer-Rochow VB (2011) Afferent and efferent pathways in the visceral system of the freshwater snail Planorbarius corneus. Zool Res 32:403–420
van Duivendoden YA (1982) Non-ocular photoreceptors and photo-orientation in the pond snail Lymnaea stagnalis. J Comp Physiol 149:363–368
Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN (2010) Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468:921–926
Zhukov VV (2007) On the problem of retinal transmitters of the freshwater mollusc Lymnaea stagnalis. J Evol Biochem Physiol 43:524–532
Acknowledgments
We thank Suguru Kobayashi for his helpful advice on the electrophysiological experiments, and Takeshi Morita for his help in the measurement of the irradiance of light. This study was supported by Grants-in-Aid for KAKENHI from the Japan Society for the promotion of science (No. 25440181 to RM), the Yamada Science Foundation (to RM), and the Naito Foundation (to RM). We minimized the number of animals used, and reduced the pain of the animals by deep anesthesia (with cold Mg2+ buffer) when sacrifice.
Conflict of interest
The authors have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuo, Y., Uozumi, N. & Matsuo, R. Photo-tropotaxis based on projection through the cerebral commissure in the terrestrial slug Limax . J Comp Physiol A 200, 1023–1032 (2014). https://doi.org/10.1007/s00359-014-0954-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0954-7