Abstract
Objectives
The preoperative prediction of the WHO grade of a meningioma is important for further treatment plans. This study aimed to assess whether texture analysis (TA) based on apparent diffusion coefficient (ADC) maps could non-invasively classify meningiomas accurately using tree classifiers.
Methods
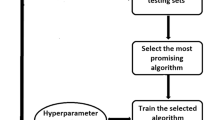
A pathology database was reviewed to identify meningioma patients who underwent tumour resection in our hospital with preoperative routine MRI scanning and diffusion-weighted imaging (DWI) between January 2011 and August 2017. A total of 152 meningioma patients with 421 preoperative ADC maps were included. Four categories of features, namely, clinical features, morphological features, average ADC values and texture features, were extracted. Three machine learning classifiers, namely, classic decision tree, conditional inference tree and decision forest, were built on these features from the training dataset. Then the performance of each classifier was evaluated and compared with the diagnosis made by two neuro-radiologists.
Results
The ADC value alone was unable to distinguish three WHO grades of meningiomas. The machine learning classifiers based on clinical, morphological features and ADC value could achieve equivalent diagnostic performance (accuracy = 62.96%) compared to two experienced neuro-radiologists (accuracy = 61.11% and 62.04%). Upon analysis, the decision forest that was built with 23 selected texture features and the ADC value from the training dataset achieved the best diagnostic performance in the testing dataset (kappa = 0.64, accuracy = 79.51%).
Conclusions
Decision forest with the ADC value and ADC map-based texture features is a promising multiclass classifier that could potentially provide more precise diagnosis and aid diagnosis in the near future.
Key Points
• A precise preoperative prediction of the WHO grade of a meningioma brings benefits to further treatment plans.
• Machine learning models based on clinical, morphological features and ADC value could achieve equivalent diagnostic performance compared to experienced neuroradiologists.
• The decision forest model built with 23 selected texture features and the ADC value achieved the best diagnostic performance (kappa = 0.64, accuracy = 79.51%).





Similar content being viewed by others
Abbreviations
- TA:
-
Texture analysis
- ADC:
-
Apparent diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- ROI:
-
Region of interest
References
Ostrom QT, Gittleman H, Liao P et al (2017) CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 19(suppl_5):v1–v88
Nabors LB, Portnow J, Ammirati M et al (2017) NCCN Guidelines Insights: Central Nervous System Cancers, Version 1.2017. J Natl Compr Canc Netw 15:1331–1345
Champeaux C, Dunn L (2016) World Health Organization grade ii meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg 89:180–186
Rogers L, Barani I, Chamberlain M et al (2015) Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 122:4–23
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Champeaux C, Houston D, Dunn L (2017) Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie 63:273–281
Aizer AA, Bi WL, Kandola MS et al (2015) Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 121:4376–4381
Moliterno J, Cope WP, Vartanian ED et al (2015) Survival in patients treated for anaplastic meningioma. J Neurosurg 123:23–30
Gutman DA, Dunn WD, Grossmann P et al (2015) Somatic mutations associated with MRI-derived volumetric features in glioblastoma. Neuroradiology 57:1227–1237
Svolos P, Tsolaki E, Theodorou K et al (2013) Classification methods for the differentiation of atypical meningiomas using diffusion and perfusion techniques at 3-T MRI. Clin Imaging 37:856–864
Yin B, Liu L, Zhang BY, Li YX, Li Y, Geng DY (2012) Correlating apparent diffusion coefficients with histopathologic findings on meningiomas. Eur J Radiol 81:4050–4056
Lu Y, Xiong J, Yin B, Wen J, Liu L, Geng D (2018) The role of three-dimensional pseudo-continuous arterial spin labelling in grading and differentiating histological subgroups of meningiomas. Clin Radiol 73:176–184
Surov A, Gottschling S, Mawrin C et al (2015) Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. Transl Oncol 8:517–523
Tang Y, Dundamadappa SK, Thangasamy S et al (2014) Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am J Roentgenol 202:1303–1308
Vermoolen MA, Kwee TC, Nievelstein RAJ (2012) Apparent diffusion coefficient measurements in the differentiation between benign and malignant lesions: a systematic review. Insights Imaging 3:395–409
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Kumar V, Gu Y, Basu S et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248
Zacharaki EI, Wang S, Chawla S et al (2009) Classification of brain tumor type and grade using MRI texture and shape in a machine learning scheme. Magn Reson Med 62:1609–1618
Kassner A, Thornhill RE (2010) Texture analysis: a review of neurologic MR imaging applications. AJNR Am J Neuroradiol 31:809–816
Lessmann B, Nattkemper TW, Hans VH, Degenhard A (2007) A method for linking computed image features to histological semantics in neuropathology. J Biomed Inform 40:631–641
Coroller TP, Bi WL, Huynh E et al (2017) Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One 12:e0187908
Yan P, Yan L, Hu T et al (2017) The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. Transl Oncol 10:570-577
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda—a software package for image texture analysis. Comp Methods Programs Biomed 94:66–76
Strzelecki M, Szczypinski P, Materka A, Klepaczko A (2013) A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instrum Methods Phys Res A 702:137–140
Barnholtz-Sloan KJS (2007) Meningiomas: causes and risk factors. Neurosurg Focus 23:E2
Rockhill J, Mrugala M, Chamberlain MC (2007) Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus 23:E1
Commins DL, Atkinson RD, Burnett ME (2007) Review of meningioma histopathology. Neurosurg Focus 23:E3
Koh DM, Padhani AR (2006) Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol 79:633–635
Azar AT, Elshazly HI, Hassanien AE, Elkorany AM (2014) A random forest classifier for lymph diseases. Comput Methods Programs Biomed 113:465–473
Naik J, Patel S (2014) Tumor detection and classification using decision tree in brain MRI. Int J Computer Sci Network Security 14:87–91
Acknowledgements
The authors thank Wang Pei, M.Sc., at Xi`an Jiaotong University, Xi`an, China, for scripting and algorithm support.
Funding
This project was supported by the National Natural Science Foundation of China (Grant No. 81471627, 81501435) and Shanghai Sailing Program (Grant No. 18YF1403000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Geng Daoying.
Conflict of interest
All authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by IRB.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Lu, Y., Liu, L., Luan, S. et al. The diagnostic value of texture analysis in predicting WHO grades of meningiomas based on ADC maps: an attempt using decision tree and decision forest. Eur Radiol 29, 1318–1328 (2019). https://doi.org/10.1007/s00330-018-5632-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5632-7