Abstract
Objectives
To investigate the cerebral structural changes related to venous erectile dysfunction (VED) and the relationship of these changes to clinical symptoms and disorder duration and distinguish patients with VED from healthy controls using a machine learning classification.
Methods
45 VED patients and 50 healthy controls were included. Voxel-based morphometry (VBM), tract-based spatial statistics (TBSS) and correlation analyses of VED patients and clinical variables were performed. The machine learning classification method was adopted to confirm its effectiveness in distinguishing VED patients from healthy controls.
Results
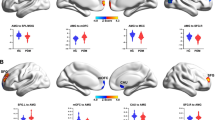
Compared to healthy control subjects, VED patients showed significantly decreased cortical volumes in the left postcentral gyrus and precentral gyrus, while only the right middle temporal gyrus showed a significant increase in cortical volume. Increased axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) values were observed in widespread brain regions. Certain regions of these alterations related to VED patients showed significant correlations with clinical symptoms and disorder durations. Machine learning analyses discriminated patients from controls with overall accuracy 96.7%, sensitivity 93.3% and specificity 99.0%.
Conclusions
Cortical volume and white matter (WM) microstructural changes were observed in VED patients, and showed significant correlations with clinical symptoms and dysfunction durations. Various DTI-derived indices of some brain regions could be regarded as reliable discriminating features between VED patients and healthy control subjects, as shown by machine learning analyses.
Key Points
• Multimodal magnetic resonance imaging helps clinicians to assess patients with VED.
• VED patients show cerebral structural alterations related to their clinical symptoms.
• Machine learning analyses discriminated VED patients from controls with an excellent performance.
• Machine learning classification provided a preliminary demonstration of DTI’s clinical use.





Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- BPRS:
-
Brief Psychiatric Rating Scale
- FA:
-
Fractional anisotropy
- GM:
-
Grey matter
- HAMA:
-
Hamilton Anxiety Rating Scale
- HAMD:
-
Hamilton Depression Rating Scale
- IIEF-5:
-
International Index of Erectile Function
- MD:
-
Mean diffusivity
- NIH-CPSI:
-
National Institutes of Health Chronic Prostatitis Symptom Index
- PED:
-
Psychogenic ED
- PEDT:
-
Premature Ejaculation Diagnostic Tool
- RD:
-
Radial diffusivity
- SAS:
-
Self-Rating Anxiety Scale
- SDS:
-
Self-Rating Depression Scale
- TBSS:
-
Tract-based spatial statistics
- VBM:
-
Voxel-based morphometry
- VED:
-
Venous erectile dysfunction
- WM:
-
White matter
References
Wespes E, Amar E, Hatzichristou D et al (2006) EAU Guidelines on Erectile Dysfunction: An Update. European Urology 49:806–815
Fabbri A, Caprio M, Aversa A (2003) Pathology of erection. Journal of endocrinological investigation 26:87–91
Vicenzini E, Altieri M, Michetti PM et al (2008) Cerebral vasomotor reactivity is reduced in patients with erectile dysfunction. European neurology 60:85–88
Rajkumar RP (2015) The impact of disrupted childhood attachment on the presentation of psychogenic erectile dysfunction: An exploratory study. The journal of sexual medicine 12:798–803
Glina S, Cohen DJ, Vieira M (2014) Diagnosis of erectile dysfunction. Current opinion in psychiatry 27:394–399
Argiolas A, Melis MR (2005) Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Progress in neurobiology 76:1–21
Stoléru S, Fonteille V, Corneill C, Joyal C, Moulier V (2012) Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neuroscience & Biobehavioral Reviews 36:1481–1509
Redoutc J, Stolutc S, Pugeat M et al (2005) Brain processing of visual sexual stimuli in treated and untreated hypogonadal patients. Psychoneuroendocrinology 30:461–482
Montorsi F, Perani D, Anchisi D et al (2003) Brain activation patterns during video sexual stimulation following the administration of apomorphine: results of a placebo-controlled study. European Urology 43:405–411
Mouras H, Stolasa S, Bittoun J et al (2003) Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage 20:855–869
Arnow BA, Desmond JE, Banner LL et al (2002) Brain activation and sexual arousal in healthy, heterosexual males. Brain 125:1014–1023
Cera N, Delli Pizzi S, Di Pierro E, Gambi F, Tartaro A, Zang Y-F (2012) Macrostructural Alterations of Subcortical Grey Matter in Psychogenic Erectile.
Zhang P, Liu J, Li G et al (2014) White matter microstructural changes in psychogenic erectile dysfunction patients. Andrology 2:379–385
Zhao L, Guan M, Zhang X et al (2015) Structural insights into aberrant cortical morphometry and network organization in psychogenic erectile dysfunction. Human brain mapping 36:4469–4482
Zhao L, Guan M, Zhu X et al (2015) Aberrant topological patterns of structural cortical networks in psychogenic erectile dysfunction. Frontiers in human neuroscience 9
Ferretti A, Caulo M, Del Gratta C et al (2005) Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage 26:1086–1096
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. Neuroimage 11:805–821
Ashburner J (2012) SPM: a history. Neuroimage 62:791–800
Arrigo A, Calamuneri A, Milardi D et al (2017) Visual System Involvement in Patients with Newly Diagnosed Parkinson Disease. Radiology:161732
Fan W, Zhang W, Li J et al (2015) Altered contralateral auditory cortical morphology in unilateral sudden sensorineural hearing loss. Otology & Neurotology 36:1622
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Smith SM (2002) Fast robust automated brain extraction. Human brain mapping 17:143–155
Pedregosa F, Varoquaux G, Gramfort A et al (2011) Scikit-learn: Machine learning in Python. Journal of Machine Learning Research 12:2825–2830
Mori S, Oishi K, Jiang H et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582
Poeppl TB, Langguth B, Laird AR, Eickhoff SB (2014) The functional neuroanatomy of male psychosexual and physiosexual arousal: A quantitative meta-analysis. Human brain mapping 35:1404–1421
Kader KO, Sanverdi E, Has A, Temuçin Ç, T mu S, Doerschner K (2013) Tract-based spatial statistics of diffusion tensor imaging in hereditary spastic paraplegia with thin corpus callosum reveals widespread white matter changes. Diagnostic and Interventional Radiology 19:181
Alexander AL, Hurley SA, Samsonov AA et al (2011) Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain connectivity 1:423–446
Song S-K, Yoshino J, Le TQ et al (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magnetic Resonance in Medicine 55:302–308
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329
Rouw R, Scholte HS (2007) Increased structural connectivity in grapheme-color synesthesia. Nature neuroscience 10:792
Scholz J, Klein MC, Behrens TE, Johansen-Berg H (2009) Training induces changes in white-matter architecture. Nature neuroscience 12:1370–1371
Jellinger K (2007) Fiber Pathways of the Brain. European Journal of Neurology 14
Henze R, Brunner R, Thiemann U et al (2012) White matter alterations in the corpus callosum of adolescents with first-admission schizophrenia. Neuroscience letters 513:178–182
Hagemann JH, Berding G, Bergh S et al (2003) Effects of visual sexual stimuli and apomorphine SL on cerebral activity in men with erectile dysfunction. European Urology 43:412–420
Acknowledgements
We would like to thank the three anonymous reviewers for their helpful comments on an earlier version of this manuscript. We thank all participants in this study.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81701673) and the Hubei Natural Science Foundation (No. 2017CFB796).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Lian Yang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained by the Medical Ethics Committee of the Union Hospital.
Methodology
• prospective
• case-control study/diagnostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOC 2877 kb)
Rights and permissions
About this article
Cite this article
Li, L., Fan, W., Li, J. et al. Abnormal brain structure as a potential biomarker for venous erectile dysfunction: evidence from multimodal MRI and machine learning. Eur Radiol 28, 3789–3800 (2018). https://doi.org/10.1007/s00330-018-5365-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5365-7