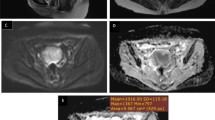
Abstract
Objective
To investigate the application value of the ADCdifference value in evaluating the pathological grade of uterine cervical cancer and to analyse the correlations among microvascular density (MVD), vascular endothelial growth factor (VEGF) expression and maximum ADCdifference value.
Methods
Fifty-six patients with uterine cervical cancer were included in this prospective study. All underwent conventional MRI and DWI. MVD and VEGF were evaluated by immunohistochemical staining with anti-CD34 and anti-VEGF, respectively.
Results
Maximum ADCdifference value and MVD count showed statistical differences among different pathological grades (P < 0.001, P < 0.001). There was a significant positive linear correlation between the maximum ADCdifference value and pathological tumour grade (P < 0.001), and also between MVD count and pathological tumour grade (P < 0.001). No significant differences were found between the level of VEGF expression and pathological tumour grade (P = 0.222). The maximum ADCdifference value correlated positively with both the MVD count and the level of VEGF expression (P < 0.001, P < 0.001).
Conclusions
Quantitative analysis of maximum ADCdifference value of uterine cervical cancer may represent the grade of tumour differentiation and provide valuable information on tumour microcirculation and perfusion, thus allowing a promising new method of non-invasively assessing the pathological grade, which could serve as a substitution for assessing tumour angiogenesis.
Key Points
• Diffusion-weighted magnetic resonance imaging offers numerous new parameters concerning cervical cancer.
• Relationships between ADC difference values and grades of tumour differentiation are examined.
• Quantitative analysis may provide valuable information on tumour microcirculation and perfusion.
• The maximum ADC difference value could serve as a substitute for assessing tumour angiogenesis.






Similar content being viewed by others
Abbreviations
- MVD:
-
microvascular density
- VEGF:
-
vascular endothelial growth factor
- DWI:
-
diffusion-weighted MR Imaging
- ASSET:
-
array spatial sensitivity encoding technique
- ADC:
-
apparent diffusion coefficient
References
Goncharuk IV, Vorobjova LI, Lukyanova NY, Chekhun VF (2009) Vascular endothelial growth factor expression in uterine cervical cancer: correlation with clinicopathologic characteristics and survival. Exp Oncol 31:179–181
Zusterzeel PL, Span PN, Dijksterhuis MG, Thomas CM, Sweep FC, Massuger LF (2009) Serum vascular endothelial growth factor: a prognostic factor in cervical cancer. J Cancer Res Clin Oncol 135:283–290
Srivastava S, Gupta A, Agarwal GG et al (2009) Correlation of serum vascular endothelial growth factor with clinicopathological parameters in cervical cancer. Biosci Trends 3:144–150
Zhang H, Pan Z, Du L et al (2008) Advanced gastric cancer and perfusion imaging using a multidetector row computed tomography: correlation with prognostic determinants. Korean J Radiol 9:119–127
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Ma SH, Le HB, Jia BH et al (2008) Peripheral pulmonary nodules: relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and VEGF expression. BMC Cancer 8:186
Bammer R (2003) Basic principles of diffusion-weighted imaging. Eur J Radiol 45:169–184
Lambregts DM, Beets GL, Maas M et al (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Li W, Chu C, Cui Y, Zhang P, Zhu M (2011) Diffusion-weighted MRI: a useful technique to discriminate benign versus malignant ovarian surface epithelial tumors with solid and cystic components. Abdom Imaging. doi:10.1007/s00261-011-9814-x
Lee MH, Kim SH, Park MJ, Park CK, Rhim H (2011) Gadoxetic acid-enhanced Hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol 197:W868–W875
Sonmez G, Cuce F, Mutlu H et al (2011) Value of diffusion-weighted MRI in the differentiation of benign and malign breast lesions. Wien Klin Wochenschr 123:655–661
Jin N, Deng J, Zhang L et al (2011) Targeted single-shot methods for diffusion-weighted imaging in the kidneys. J Magn Reson Imaging 33:1517–1525
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J (1990) Echo-planar imaging of intravoxel incoherent motion. Radiology 177:407–414
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Moore RJ, Issa B, Tokarczuk P et al (2000) In vivo intravoxel incoherent motion measurements in the human placenta using echo-planar imaging at 0.5 T. Magn Reson Med 43:295–302
Weidner N (1999) Tumour vascularity and proliferation: clear evidence of a close relationship. J Pathol 189:297–299
Colagrande S, Carbone SF, Carusi LM, Cova M, Villari N (2006) Magnetic resonance diffusion-weighted imaging: extraneurological applications. Radiol Med 111:392–419
Colagrande S, Pallotta S, Vanzulli A, Napolitano M, Villari N (2005) The diffusion parameter in magnetic resonance: physics, techniques, and semeiotics. Radiol Med 109:1–16
Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H (1999) Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 210:617–623
Wirestam R, Borg M, Brockstedt S, Lindgren A, Holtås S, Ståhlberg F (2001) Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiol 42:123–128
Callot V, Bennett E, Decking UK, Balaban RS, Wen H (2003) In vivo study of microcirculation in canine myocardium using the IVIM method. Magn Reson Med 50:531–540
Moteki T, Horikoshi H (2006) Evaluation of hepatic lesions and hepatic parenchyma using diffusion-weighted echo-planar MR with three values of gradient b-factor. J Magn Reson Imaging 24:637–645
DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K (2000) High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol 21:1830–1836
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Schoennagel BP, Habermann CR, Roesch M et al (2011) Diffusion-weighted imaging of the healthy pancreas: apparent diffusion coefficient values of the normal head, body, and tail calculated from different sets of b-values. J Magn Reson Imaging 34:861–865
Heo SH, Jeong YY, Shin SS et al (2010) Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol 11:295–303
Liu Y, Bai R, Sun H, Liu H, Wang D (2009) Diffusion-weighted magnetic resonance imaging of uterine cervical cancer. J Comput Assist Tomogr 33:858–862
Akita H, Jinzaki M, Kikuchi E et al (2011) Preoperative T categorization and prediction of histopathologic grading of urothelial carcinoma in renal pelvis using diffusion-weighted MRI. AJR Am J Roentgenol 197:1130–1136
Thoeny HC, De Keyzer F, Vandecaveye V et al (2005) Effect of vascular targeting agent in rat tumor model: dynamic contrast-enhanced versus diffusion-weighted MR imaging. Radiology 237:492–499
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Ash L, Teknos TN, Gandhi D, Patel S, Mukherji SK (2009) Head and neck squamous cell carcinoma: CT perfusion can help noninvasively predict intratumoral microvessel density. Radiology 251:422–428
Kan Z, Phongkitkarun S, Kobayashi S et al (2005) Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology 237:151–158
O’Byrne KJ, Koukourakis MI, Giatromanolaki A et al (2000) Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer 82:1427–1432
Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368–4380
Klasa-Mazurkiewicz D, Jarząb M, Milczek T, Lipińska B, Emerich J (2011) Clinical significance of VEGFR-2 and VEGFR-3 expression in ovarian cancer patients. Pol J Pathol 62:31–40
Acs G, Paragh G, Rakosy Z, Laronga C, Zhang PJ (2012) The extent of retraction clefts correlates with lymphatic vessel density and VEGF-C expression and predicts nodal metastasis and poor prognosis in early-stage breast carcinoma. Mod Pathol 25:163–177
Möbius C, Freire J, Becker I et al (2007) VEGF-C expression in squamous cell carcinoma and adenocarcinoma of the esophagus. World J Surg 31:1768–1772, discussion 1773–1774
Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM (2000) Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer 83:620–625
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Ye, Z., Sun, H. et al. Grading of uterine cervical cancer by using the ADC difference value and its correlation with microvascular density and vascular endothelial growth factor. Eur Radiol 23, 757–765 (2013). https://doi.org/10.1007/s00330-012-2657-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2657-1