Abstract
Different phenological responses to climate changes by species representing preys and predators may lead to mismatch between functionally dependent components of an ecosystem, with important effects on its structure and functioning. Here, we investigate within-season variation in zooplankton availability, chick diet composition and breeding performance of a small planktivorous seabird, the little auk (Alle alle) in two large colonies in Hornsund and Magdalenefjorden, Spitsbergen, differing in synchrony of breeding (11-day vs. 22-day hatching period, respectively). Assuming similar zooplankton phenology and existing differences in duration of the little auk breeding period, we expected lower availability of the preferred food in the less synchronized colony in Magdalenefjorden and in consequence a negative effect on nestling body mass and survival. We found that in both colonies Calanus glacialis (copepodite stage CV) was the most important prey item in the chick diet making up 68–87 % of the biomass and energy of all prey items. The only exception was the end of the chick-rearing period in Magdalenefjorden, when contribution of this prey item was significantly lower (24–26 %). Thus, late breeders in Magdalenefjorden were apparently mismatched regarding C. glacialis CV availability. However, the hatching date did not affect birds fitness (reproductive output and chick pre-fledging mass) significantly. Results of our study indicate that little auks breeding on Spitsbergen can respond to a wide range of environmental conditions and prey availabilities through the plasticity of their foraging behaviour, which may help them to maintain their optimum fitness level in changing and unpredictable environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inter- and intra-seasonal changes in environmental conditions and food availability often affect foraging behaviour and diet of animals including numerous bird species (e.g. Morrison et al. 1990; Watanuki et al. 1993; Charrassin et al. 1998; Jakubas and Manikowska 2011). Interpretation of changes in diet may differ between ecological groups. For generalists, it may indicate prey switching, i.e. increased predator selection for a particular prey species when it is abundant and decreased selection when it is scarce (e.g. Murdoch et al. 1975). For specialized predators, having strong, fixed preferences for a given prey type, regardless of its abundance (Charnov 1976), changes in diet may indicate a severe change in availability of the preferred prey, and this, in turn, may have serious negative consequences on the predator breeding performance. Thus, recognizing changes in diet of specialized predator within the breeding period may indicate changes in foraging conditions, especially in the case of predators breeding in seasonal environments. They schedule their most energy-demanding life-history events, such as reproduction to coincide with the peak of abundance of the preferred food. In many regions, however, climate changes shift annual cycles of multiple organisms (Parmesan and Yohe 2003; Root et al. 2003) and predators may respond differently than their prey. This may lead to mismatches between functionally dependent components of ecosystem with important effects on the structure and function of the whole ecosystem (Stenseth et al. 2002). The potential for mismatches between predator and prey dynamics is compounded for seabirds breeding in high latitudes. The Arctic is currently undergoing a dramatic climate change, with a twofold greater increase in temperature compared to the global increase (IPCC 2007). Moreover, high-latitude nesting seabirds have a limited time window to reproduce what may limit their ability to adjust the timing of breeding to shifts in peaks of food abundance (Svensson 1995; Shultz et al. 2009).
The little auk (or dovekie) (Alle alle) is a small, zooplanktivorous seabird breeding exclusively in the high Arctic. It is considered to be the most abundant alcid in the Palaearctic (Stempniewicz 2001). The female lays a single egg annually in a nest usually situated under boulders in mountain scree. Both partners incubate the egg, then brood (for the few first days) and feed the chick (Stempniewicz 2001). Owing to the high cost of locomotion, both in the air (flapping flight) and in the water (underwater ‘flight’), the little auk’s foraging is believed to be energetically expensive (Gabrielsen et al. 1991; Konarzewski et al. 1993). To cover such high-energy demands, the little auk forages almost exclusively on copepods associated with cold Arctic waters (in the Spitsbergen area: Calanus glacialis, especially copepodite stage CV), which are larger and much richer in energy than their counterparts from warmer Atlantic waters (C. finmarchicus) (Weslawski et al. 1999; Karnovsky et al. 2003; Jakubas et al. 2007, 2011; Fort et al. 2010; Kwasniewski et al. 2010, 2012). The distribution of cold Arctic waters along the west coast of Spitsbergen varies spatially and seasonally (Walczowski and Piechura 2007), affecting the accessibility of the zooplankton species preferred by the little auk (Kwasniewski et al. 2012). Spatial and temporal variability in ice cover extent could lead to a mismatch between the timing of ice algal and/or phytoplankton blooms and the timing of reproduction and development of C. glacialis (Søreide et al. 2010). Ice breakup occurring too early or too late can cause a mismatch between primary and secondary producers (Leu et al. 2011). For instance, early ice breakup may shorten the ice algal bloom period and also the gap between ice algal and phytoplankton blooms and hence lead to poorer growth conditions for developing C. glacialis population (Leu et al. 2011; Daase et al. 2013). Those changes may have negative consequences for the entire lipid-based Arctic marine food web (Leu et al. 2011), including top predators such as little auks.
We aimed in this study to investigate within-season variation in zooplankton availability, chick diet composition and breeding performance of little auks breeding in two large colonies on west Spitsbergen, in Hornsund and Magdalenefjorden. Both colonies are situated in the area where Atlantic, Arctic and glacial waters converge, mix and exchange (Saloranta and Svendsen 2001). The timing of ice retreat and peaks of pelagic phytoplankton bloom are similar in both areas [according to 10-year data for 1998–2007 (Ji et al. 2013) and sea ice data for the studied year (Norwegian Ice Service, http://polarview.met.no/)]. To our knowledge, there are no detailed data on zooplankton phenology of C. glacialis in the studied areas; however, given environmental conditions both studied areas probably represent Kongsfjorden phenological ice-free scenario (Daase et al. 2013). Therefore, we anticipated that C. glacialis CV abundance would also be similar in both colonies. However, given (1) descent of C. glacialis CV to deeper water starting at the beginning of August (Daase et al. 2013), (2) more prolonged breeding in Magdalenefjorden (hatching span: 25–32 days; Jakubas and Wojczulanis-Jakubas 2011) than in Hornsund (9–15 days; Stempniewicz 2001; Wojczulanis-Jakubas 2007) and (3) later start of breeding in Magdalenefjorden (Jakubas et al. 2013), we expected decreasing availability of the preferred food (C. glacialis CV) in Magdalenefjorden. As low abundance of this copepod may affect significantly energy content of the little auk chick meals (Jakubas et al. 2007), we expected that chick body mass and survival in the Magdalenefjorden colony would be lower than in Hornsund.
Methods
Study area
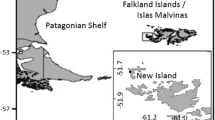
We carried out the study in two large little auk colonies in Hornsund (SW Spitsbergen; 77°00′N, 15°33′E) and Magdalenefjorden (NW Spitsbergen; 79°35′N, 11°05′E) in 2010 (Fig. 1). The two fjords are the main breeding areas of the little auk on Svalbard (Isaksen 1995). We studied the zooplankton community composition on known foraging grounds of little auks breeding in Hornsund (fjord and shelf zone) and Magdalenefjorden areas (fjord and shelf zone close to Magdalenefjorden and Smeerenburgfjorden) (Jakubas et al. 2011; 2012, 2013, 2014; Stempniewicz et al. 2013; Hovinen et al. 2014a) (Fig. 1).
(Right) Study area with ocean current patterns in the Spitsbergen region (arrows; modified after Loeng and Drinkwater 2007), sea ice extent during food sample collection in Magdalenefjorden (based on ice maps from the Norwegian Ice Service; http://polarview.met.no/) and the locations and sizes of little auk colonies (circles, data of Norwegian Polar Institute). (Left) Close-up of the Magdalenefjorden (M), Smeerenburgfjorden (S) (upper) and Hornsund (H) areas (lower), showing zooplankton sampling sites (circles and triangles), and little auk colonies (the little auk icon); K Kongsfjorden
The Hornsund area is influenced by both the coastal Sørkapp Current, carrying cold, less saline Arctic-type water from the northeast Barents Sea, and the West Spitsbergen Current (WSC), supplying warmer and more saline Atlantic waters from the Norwegian Sea (Piechura et al. 2001). In the vicinity of Magdalenefjorden, the WSC flows over the shelf slope and meets relatively cold and fresh Arctic water present there in the form of an anticyclonic coastal current. The two water masses partly mix, creating a transitional zone of differing widths depending on the season (Daase et al. 2007; Walczowski and Piechura 2007; Trudnowska et al. 2012). Smeerenburgfjorden lies at the northern end of the cold coastal current that flows along the west coast of Spitsbergen, but it is also strongly influenced by the Svalbard Branch of WSC (Saloranta and Svendsen 2001; Walczowski et al. 2005), which carries warm, saline Atlantic water to the north of the island. During the little auk breeding season in the studied year, the marginal sea ice zone was located 110–190 km north off Magdalenefjorden, and there was no ice in the Hornsund area (Norwegian Ice Service; http://polarview.met.no/) (Fig. 1).
Field work
We took the zooplankton samples from each of the distinguished little auk foraging grounds twice during the whole chick-rearing period—32 and 6 samples in the Hornsund area (hereafter H1–H2), 6 and 15 in the Magdalenefjorden area (M1–M2), and 6 and 15 in the Smeerenburgfjorden area (S1–S2) (for sampling dates—see Fig. 2). Sampling was carried out from the research vessel Oceania (Institute of Oceanology, Polish Academy of Sciences), except for the first sampling in the Magdalenefjorden/Smeerenburgfjorden area (M1 and S1) that was done from the Norwegian research vessel Lance (Norwegian Polar Institute). Different number of samples collected in particular periods and sites were determined by weather conditions during the sampling and logistic reasons. A WP-2-type net with a 0.25-m2 opening area (Tranter and Fraser 1968) and fitted with filtering gauze of 500 μm mesh size was hauled vertically from 50 m depth to the surface (considering maximal dive depth of 38 m attained by depth recorder equipped little auks, Karnovsky et al. 2011). We preserved the zooplankton samples in 4 % formaldehyde solution in borax-buffered seawater.
Timing of data collection during the field work in Spitsbergen, 2010. The graphs above and below the diagram show the little auk hatching dynamics in Magdalenefjorden (grey line, up) and Hornsund (black line, bottom). Bolded numbers in white bars indicate median hatching dates at the whole colony scale. The numbers in grey bars denote the chick age for early (EB; 1–50 % of the hatching date distribution) and late (LB; 51–100 % of the hatching date distribution) breeders; days marked in black in grey bars indicate peak body mass days in nests of early and late breeders (based on data from Table 7). Duration of chick presence in nests in Hornsund and Magdalenefjorden according to Harding et al. (2004) and Wojczulanis-Jakubas and Jakubas (2012), respectively. Codes for food sampling in colonies in Magdalenefjorden: MC1–MC4 and in Hornsund: HC1–HC3. Codes for zooplankton sampling in the little auks’ foraging areas in Magdalenefjorden: M1–M2, in Smeerenburgfjorden: S1–S2 for, and in Hornsund: H1–H2
We took samples of the food loads from adult little auks carrying food to their chicks captured in the colony with mist nets or noose carpets. We caught birds in two colony patches in Magdalenefjorden estimated to 1100 and 5000 breeding pairs, respectively, and in one patch in Hornsund (estimated to 3240 breeding pairs; Keslinka et al., unpublished data). We gently scooped the food content out of the little auk’s gular pouch with a small spoon and released the birds after 5–10 min of handling. We put each food load into a separate plastic container and preserved it in 4 % formaldehyde solution in seawater. We collected food samples three times in Hornsund (hereafter HC1–HC3, N = 13, 23 and 13) and four times in Magdalenefjorden (MC1–MC4, N = 23, 23, 19 and 23) (for sampling dates—see Fig. 2).
To establish hatching dates, we inspected 126 nests in Hornsund and 128 in Magdalenefjorden. Active nests were found during incubation and checked every 2 day, starting from 2 d before the expected hatching date (estimated according to data from previous seasons) until we found a hatchling. Then, to investigate chick survival and chick growth, we monitored the nests (N = 78 and 94 in Hornsund and Magdalenefjorden, respectively) starting from when the chicks were at age 14–15 days. We weighed chicks every 3 days until they disappeared from the nest at 21–31 day (Harding et al. 2004; Wojczulanis-Jakubas and Jakubas 2012). We did not capture the chicks for the first 2 weeks of their life to minimize disturbance and considered the hatched chicks that were not found at 14–15 days as dead. We weighed the chicks to the nearest 0.1 g using a 300 g OHAUS electronic balance. Many little auk nesting chambers are difficult to access and/or chicks are able to escape from the main chamber when disturbed, which influenced particular sample sizes. Thus, sample sizes varied for hatching dynamic, survival and chick growth (Table 7).
Data analyses
We calculated zooplankton dry masses according to Kwasniewski et al. (2010) and literature therein. We calculated the energy estimates according to Harris et al. (2000).
We applied the following multivariate methods to investigate spatio-temporal variations in the qualitative and quantitative composition of zooplankton samples collected on the foraging grounds and from birds: (1) non-metric multidimensional scaling (nMDS), an indirect gradient analysis approach which produces an ordination based on a distance matrix explained by the Bray–Curtis similarity measure (Taguchi and Oono 2005), used to visualize similarity of foraging ground zooplankton communities and food load compositions (shown only for biomass); 2) analysis of group similarities (ANOSIM), a procedure based on Bray–Curtis measure of similarity to test differences among groups (Clarke 1993); 3) the similarity percentage breakdown (SIMPER) procedure, to assess the average per cent contribution of individual items to the dissimilarity between objects in a Bray–Curtis dissimilarity matrix; significance of results was assessed by ANOSIM (Clarke 1993). Furthermore, we applied the nonparametric Kruskal–Wallis test to compare abundance, biomass and energy content of Calanus glacialis and all prey items combined between the samples from foraging grounds and from the little auk chick diet.
We deciphered and visualized spatial and temporal differences in the diet using a feeding strategy Costello diagram. It incorporates prey-specific abundance (i.e. the percentage a prey taxon comprises of all prey items in only those samples in which the actual prey occurs) and frequency of occurrence metrics to indicate prey importance, dominant or rare and the predator feeding strategy (specialist or generalist) (Costello 1990, modified by Amundsen et al. 1996).
To compare distribution of hatching dates between the colonies, we used Mann–Whitney U test. To compare chick growth between the colonies, we used the following variables: peak mass (the highest mass noted per chick), the day when the peak mass was achieved (Stempniewicz 1980) and pre-fledging mass (at 21–22 days). The peak and fledging masses have been found to be effective growth indicators (Zach 1988). Because the study was completed before majority of chicks fledged, we used in analyses pre-fledging mass.
To estimate the effect of colony, hatching date and their interaction on chick growth variables (i.e. peak mass, peak day mass and pre-fledging mass), we used general linear model (GLM). Nonsignificant interaction terms (P > 0.05) were removed and analyses rerun including only colony and hatching date.
To investigate breeding success between the two colonies, we analysed chick survival up to 20 days (the number of 20-day-old chicks/number of chicks hatched; chicks that disappeared from the nest after 20 days were assumed to have fledged; Harding et al. 2004). We analysed data on chick survival using logistic regression starting with models that included hatching date as continuous independent variable, colony as the grouping variable, and the interaction between the colony and hatching date. If the interaction term was nonsignificant (P > 0.05) in the first run, we removed it and rerun the analysis including only hatching date and colony terms.
To visualize differences in phenology between early and late breeders, we presented duration of chick presence in nests [according to Harding et al. (2004) in Hornsund and Wojczulanis-Jakubas and Jakubas (2012) in Magdalenefjorden] of early (chicks hatched on dates representing a first half, i.e. 1–50 % of the hatching date distribution) and late (chicks hatched on dates representing a second half, i.e. 51–100 % of the hatching date distribution) breeders (Figs. 1, 5).
We performed nMDS, ANOSIM and SIMPER procedures using PAST 3.0 software (Hammer et al. 2001). We expressed results as similarities in nMDS and ANOSIM and as dissimilarities in SIMPER. All other analyses we conducted in STATISTICA 8.0 (StatSoft, Inc., Tulsa, Oklahoma, USA) and R software (R Core Team 2015).
Results
Zooplankton structure on the foraging grounds
In Magdalenefjorden, abundance and biomass of all zooplankton items combined during the first sampling in Magdalenefjorden (M1) were significantly higher compared to the second sampling (M2). M1 and M2 values were significantly lower compared to both Hornsund samplings (H1–H2), and last sampling in Smeerenburgfjorden (S2) (Table 1). In Magdalenefjorden, abundance and biomass of C. glacialis CV collected during the second sampling (M2) were significantly lower compared to the first one (M1), as well as to the second sampling in Hornsund (H2). In Hornsund, values recorded there during the second sampling (H2) were significantly higher compared to H1. In Smeerenburgfjorden, abundance and biomass of C. glacialis CV were similar in both samplings (Table 1; Fig. 3a).
nMDS plots of Bray–Curtis similarities in the relative biomass of zooplankton taxa in a samples collected in the little auk foraging grounds in Magdalenefjorden (M1–M2) and Hornsund (H1–H2) shelf zones, in Smeerenburgfjorden (S1–S2) and b in the food samples collected from little auks in the colonies in Hornsund (HC1–HC3) and Magdalenefjorden (HC1–HC4) in 2010. Convex hulls contain all samples from one area/date
The nMDS plot of similarity in zooplankton composition regarding its biomass showed no clear separation between various areas/dates; however, samples M2 and H2 generally clustered in two positions different from the rest of the samples (Fig. 3a). These samples were characterized by the lowest (M2) and the highest (H2) biomass of the little auk most important prey, C. glacialis CV (Table 1).
SIMPER procedure showed that samples from the little auk foraging grounds in the Magdalenefjorden (average within-group dissimilarity: abundance 82 %, biomass 77 %) and Hornsund areas (average within-group dissimilarity: abundance 78 %, biomass 83 %) were more dissimilar than the samples from Smeerenburgfjorden (average within-group dissimilarity: abundance 58 %, biomass 61 %) (Table 2). SIMPER also showed a high degree of zooplankton dissimilarity between the second sampling in Magdalenefjorden (M2) and all other sampling areas (abundance 79–93 %, biomass 78–93 %). Analyses also revealed a high degree of zooplankton dissimilarity between the two samplings in the Hornsund area (H1 and H2; biomass 83 %) and the second in the Smeerenburgfjorden area (S2; biomass 84 %) (Table 2).
The SIMPER analysis showed that C. finmarchicus CIII and CIV development stages contributed the most (24 and 39 %, respectively) to the observed pattern of dissimilarity in abundance. Stages CIV and CV of this copepod made substantial contributions (33 and 17 %, respectively) to the observed dissimilarities in biomass (Table 3).
Chick diet composition
The nMDS plot of similarity in the diet composition (by biomass) showed no clear separation among colonies and dates. However, many MC4 samples were clustered in one group, different from the others (Fig. 3b). Generally, the diet samples from Hornsund were clustered more closely together than those from Magdalenefjorden (Fig. 3b). SIMPER procedure revealed that samples from the Magdalenefjorden colony were more dissimilar (average within-group dissimilarity: abundance 60 %, biomass and energy content 61 %) than those from the Hornsund colony (average within-group dissimilarity: abundance 29 %, biomass and energy content 30 %) (Table 4). SIMPER analysis also showed a high degree of dissimilarity in dietary composition between the last sample collected in Magdalenefjorden (MC4) and other samples (abundance: 79–83 %, biomass and energy content 82–85 %) (Table 4). SIMPER analysis showed that C. glacialis CV contributed greatly (59–64 %) to the observed dissimilarity in abundance, biomass and energy content among diet samplings. The sympagic amphipod Apherusa glacialis contributed 8 % to the observed dissimilarity in biomass and energy content (Table 3).
Comparison of abundance, biomass (dry mass) and energy content of C. glacialis CV in food samples revealed significantly lower values in MC4 compared to all other samplings. The medians were 46–58 times lower compared to earlier samplings in Magdalenefjorden and 45–65 times lower compared to Hornsund. In the case of all diet items combined, significant differences between MC4 and the majority of other samplings were recorded with median values 4–6 (abundance) and 2–3 times (biomass and energy) lower than in earlier samplings in Magdalenefjorden and 4–5 (abundance) and 2–3 (biomass and energy) times lower than in Hornsund (Table 5; Fig. 3b).
Dietary composition was generally similar in both colonies at all stages of the nestling period. C. glacialis CV was dominant prey, occurring frequently, and taken in great numbers (Fig. 4). Ancillary prey occurred in samplings MC2 and MC4 from Magdalenefjorden and included A. glacialis. The last sampling in Magdalenefjorden (MC4) was exceptional—there was no dominant prey species; the majority of prey items (including C. glacialis CV) were classified as being taken occasionally by most birds (Fig. 4).
Costello diagrams, i.e. scatterplots of all little auk prey species according to their occurrence and prey-specific abundance in diet samples collected in different periods in Hornsund (HC1–HC3) and Magdalenefjorden (MC1–MC4) in 2010. Lower left Interpretation of the diagram according to Amundsen et al. (1996)
The frequency of occurrence of C. glacialis CV in MC4 food loads was lower (24 % and 26 % in terms of biomass and energy, respectively) compared to other samplings (up to 87 %) (Table 6). A. glacialis made up 36 and 38 % of all prey items in terms of biomass and energy content, respectively (Table 6).
Hatching phenology, chick survival and body mass
The distributions of hatching dates were similar in both colonies (Table 7; Fig. 2; Mann–Whitney U test, Z 128, 126 = 1.73, P = 0.08) with median values on 15 and 16 July in Hornsund and Magdalenefjorden, respectively. However, the hatching period in Magdalenefjorden lasted twice as long as in Hornsund (Table 7; Fig. 2).
In the logistic regression for chick survival up to 20 d, the hatching date x colony interaction term was insignificant (P = 0.94). In analysis excluding the interaction, neither hatching date (P = 0.22) nor colony (P = 0.81) affected chick survival significantly. Chick survival up to 20 d was high (>95 %) and did not differ significantly between the two colonies (Table 7).
None of the GLM analyses (for peak body mass, pre-fledging mass and peak body mass age) revealed significant interaction between the hatching date and colony (all P > 0.29). This indicates that the slopes relating those variables to hatching date did not differ strongly between the colonies. When we excluded the interaction term from the model, the colony affected significantly peak body mass (P = 0.002); chicks in Hornsund were significantly heavier (by 7.1 g) compared to Magdalenefjorden (Table 7). Hatching date did not affect peak body mass significantly (P = 0.91). Age at which the chicks gained peak body mass was affected significantly by the colony (P = 0.02); chicks in Hornsund reached their peak body mass 1 day earlier than in Magdalenefjorden (Table 7). Hatching date did not affect peak body mass age significantly (P = 0.51). The pre-fledging chick body mass was affected neither by colony (P = 0.25) nor by hatching date (P = 0.84) (Table 7).
Discussion
To our knowledge, this is the first study investigating within-season variation in zooplankton availability, chick diet composition and breeding performance of zooplanktivorous little auks. Studies on changes in diet of zooplanktivorous seabirds may be especially informative, because they feed on organisms that respond relatively quickly and predictably to environmental variations (Bertram et al. 2001; Sydeman et al. 2001; Hipfner 2009).
Previous papers have shown that Calanus glacialis CV is the most important prey item for little auks breeding on Svalbard (Weslawski et al. 1999; Karnovsky et al. 2003; Kwasniewski et al. 2010; Jakubas et al. 2007, 2011, 2016, Kidawa et al. 2015). Our study indicates that this preference is temporally consistent throughout the chick-rearing period. This prey item made up 68–87 % of all dietary components in terms of biomass and energy in both colonies during all the samplings in Hornsund and the first three in Magdalenefjorden. Only during the last diet sampling in Magdalenefjorden (MC4), contribution of C. glacialis CV was considerably lower (24 and 26 % in biomass and energy content, respectively), suggesting a mismatch of late breeders in this colony with the peak availability of the preferred prey item.
Considering similar ice conditions (i.e. lack of ice in close proximity of colonies) in both areas and high biomass of C. glacialis CV in zooplankton samples collected in Hornsund at the same time as MC4 chick diet sampling in Magdalenefjorden, one may expect similar C. glacialis CV availability for birds from both colonies, even late in the season. Although we have no data on zooplankton abundance in the little auk feeding grounds for the period of the last diet sampling in the Magdalenefjorden colony (MC4), our results suggest divergent patterns of C. glacialis CV availability in both studied areas throughout the little auk nestling period. Firstly, the biomass of that prey item found in the zooplankton samples collected in the Magdalenefjorden area (M2) 1 week before the last diet sampling (MC4) was significantly lower than during the first sea sampling there (M1) (Fig. 5). Also, the biomass of C. glacialis CV in M2 sample was significantly lower than that found at the same phenological stage in the Hornsund area (H2) (Fig. 5). Secondly, we found an opposite pattern of changes in C. glacialis CV abundance and biomass between the first and second sea samplings in the two areas, with an increasing trend in Hornsund and decreasing one in Magdalenefjorden feeding grounds. This indicates a poor availability of this prey item at the end of chick-rearing phase in the latter colony during MC4 diet sampling suggesting mismatch of late breeders with peak availability of the preferred food. It suggests that abundance of C. glacialis CV throughout the little auk chick-rearing period might differ between areas despite similar winter and spring ice conditions determining the timing of ice algal and phytoplankton blooms and subsequently the timing of life-history events of this copepod (Daase et al. 2013). Differences in specific local conditions (e.g. temperature, timing of the onset and magnitude of the spring phytoplankton bloom) may affect development and recruitment of C. glacialis in both areas (Daase et al. 2013). Additionally, a study of fine-scale zooplankton vertical distribution performed at the turn of July and August 2010 revealed that in the shelf zone in Hornsund area the highest proportion of C. glacialis to C. finmarchicus was found at the surface layer (depth of 0–10 m) contrary to the Magdalenefjorden area, where it was recorded considerably deeper (20–40 m) (Trudnowska et al. 2015). Moreover, autumn descent of C. glacialis CV to deeper water (<50 m) in Kongsfjorden (75 km south of Magdalenefjorden, see Fig. 1) starts at the beginning of August (Daase et al. 2013). Thus, late breeders in Magdalenefjorden may face lowered availability of the preferred food at the surface water layer late in the season forcing them to forage in different feeding areas or on the traditional feeding grounds but in suboptimal trophic conditions.
Biomass of C. glacialis CV (medians), in the zooplankton samples collected on the foraging grounds in the Magdalenefjorden (M1–M2) (a) and Hornsund (H1–H2) (b), in Smeerenburgfjorden (S1–S2) (a) (black bars, left y axis), and in the food samples collected in the colonies in Magdalenefjorden (a) (MC1–MC4) and Hornsund (b) (HC1–HC3) in 2010 (grey bars, right y axis); the horizontal lines with numbers in frames above graphs indicate nesting period duration with particular days of chicks’ lives in Magdalenefjorden (light grey) and in Hornsund (dark grey) in 2010; duration of nestling period according to literature data (Harding et al. (2004) for Hornsund, and Wojczulanis-Jakubas and Jakubas (2012) for Magdalenefjorden) for nests of early (EB; 1–50 % of hatching date distribution) and late (LB; 51–100 % of hatching date distribution) breeders; days marked in black indicate peak body mass days (see Table 7)
Seabirds can respond to decreasing availability of the preferred prey by making a dietary switch. For example, the zooplanktivorous Cassin’s auklets Ptychoramphus aleuticus fed their offspring with more Neocalanus cristatus early in the season, but with more Thysanoessa spp. later in the breeding period (Hipfner 2009). The last food samples from Magdalenefjorden (MC4) contained a large proportion of alternative energy-rich prey items like Apherusa glacialis or Calanus hyperboreus, which suggests that the little auks explored more distant foraging grounds. These prey items occur in substantial densities relatively far from Magdalenefjorden, in the marginal sea ice zone (ca 110–140 km from the colony at that time, Fig. 1) and in the open sea in the West Spitsbergen Current (Lønne and Gulliksen 1991; Scott et al. 1999; Arndt and Swadling 2006; Hop et al. 2006; Kwasniewski et al. 2010). The regular presence of the ice-associated amphipod A. glacialis in food samples from Magdalenefjorden has been reported earlier (Kwasniewski et al. 2010; Jakubas et al. 2011; Boehnke et al. 2015), and GPS tracking has confirmed that little auks forage repeatedly in the marginal sea ice zone (Jakubas et al. 2012, 2013). Such a switch between foraging grounds was not fully effective; the total energy content of the MC4 food loads was 2–3 times less than earlier samples from the same colony. Given the high costs of little auk locomotion and foraging (Gabrielsen et al. 1991; Konarzewski et al. 1993), it seems that frequent exploitation of distant feeding grounds over a longer time scale could be too costly for the parent birds. In the course of chick-rearing period, they normally reach such distant foraging areas only during infrequent long foraging trips (Jakubas et al. 2012).
In contrast to the planktivorous Cassin’s auklet (in which decreased nestling growth and survival have been reported in ‘mismatch’ years with reduced prevalence and persistence of its primary prey, N. cristatus in nestling diets; Hipfner 2008), low-energy food with a very small proportion of the preferred C. glacialis CV delivered to chicks in Magdalenefjorden late in the season (MC4) did not adversely affect little auk nestlings. Peak body mass was higher in Hornsund; however, it was not affected by the hatching date. The pre-fledging body mass and chick survival up to 20 days were similar in both colonies and were not related to the hatching date. Given the advanced age of chicks of the late breeders’ in Magdalenefjorden during the ‘mismatched’ MC4 sampling (25–26 days of life; Figs. 2, 5), they may have been little affected by the smaller quantity and inferior quality of food loads, since they were already in the phase of decreasing body mass after reaching the peak mass around 20 d of life (Stempniewicz 1980) and their energy requirements were relatively low at that stage of development (Konarzewski et al. 1993). Moreover, at that age little auk chicks usually have the highest fasting capacity owing to the considerable extra fat reserves they have built up (Taylor and Konarzewski 1989). However, worse quality and quantity of food delivered to chicks of late breeders late in the season may have affected their date of fledging. We have no data to verify it but slower chick growth rate in suboptimal food condition has been documented in the zooplanktivorous Cassin’s auklet (Bertram et al. 2001, Hedd et al. 2002) as well in the little auk in the studied colonies in previous seasons (chicks from Hornsund fledged earlier than in Magdalenefjorden; Jakubas et al. 2013).
Our results show that timing of breeding of little auks generally matched high availability of their preferred prey in both colonies in the year of this study, except for the late breeders in the Magdalenefjorden colony, which probably were mismatched during the late stage of chick-rearing period. Despite low-energy content of food delivered to chicks late in the season (during our last sampling) in Magdalenefjorden, reproductive output was not affected significantly by the hatching date. This confirms previous observations that little auks breeding on Spitsbergen can respond to a wide range of environmental conditions and prey availabilities through the plasticity of their foraging behaviour (Jakubas et al. 2007, 2011, 2013, 2016; Harding et al. 2009; Kwasniewski et al. 2010). The effectiveness of behavioural buffering may be affected by oceanographic conditions and colony location. Hovinen et al. (2014b) revealed that fledging probability was generally higher in Magdalenefjorden (range 0.91–1.0) with lower sea surface temperature (SST) than in Isfjorden (range 0.62–0.97) with higher SST, resulting in worse prey availability and quality in the little auk’s foraging grounds. Location of colony in area offering alternative foraging grounds, which may be explored when the trophic conditions in the traditional foraging areas deteriorate, may determine the birds’ ability to modify foraging strategy (Jakubas et al. 2012, 2016; Kidawa et al. 2015).
We are aware of some limitations of our study. Areas of at-sea sampling were chosen based on previous knowledge of little auks foraging grounds location, although we cannot exclude that birds sometimes would have foraged outside those traditional areas, e.g. in local temporary phenomena, like eddies enhancing productivity and concentration of zooplankton (Joiris and Falck 2011; Jakubas et al. 2013). Our limited data set based on one year and two zooplankton sampling replicates is insufficient for a comprehensive test of the match–mismatch hypothesis. The data presented here can, however, be treated as a pilot study contributing to the planning of a broad-scaled investigation covering several seasons and sites differing in oceanographic conditions and zooplankton/bird phenology. Nevertheless, our study has filled an evident gap in knowledge about within-season diet variation of the little auk and its preferred food availability in the high Arctic.
References
Amundsen PA, Gabler HM, Staldvik FJ (1996) A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. J Fish Biol 48:607–614
Arndt CE, Swadling KM (2006) Crustacea in Arctic and Antarctic sea ice: distribution, diet and life history strategies. Adv Mar Biol 51:197–315
Bertram DF, Mackas DL, McKinnell SM (2001) The seasonal cycle revisited: interannual variation and ecosystem consequences. Prog Oceanogr 49:283–307
Boehnke R, Głuchowska M, Wojczulanis-Jakubas K, Jakubas D, Karnovsky NJ, Walkusz W, Kwasniewski S, Błachowiak-Samołyk K (2015) Supplementary diet components of the little auk chicks in two contrasting regions on the west Spitsbergen coast. Polar Biol 38:261–267
Charnov EL (1976) Optimal foraging: attack strategy of a mantid. Am Nat 110:141–151
Charrassin JB, Bost CA, Pütz K, Lage J, Dahier T, Zorn T, Le Maho Y (1998) Foraging strategies of incubating and brooding king penguins Aptenodytes patagonicus. Oecologia 114:194–201
Clarke KR (1993) Non-parametric multivariate analysis of changes in community structure. Aust J Ecol 18:117–143
Costello MJ (1990) Predator feeding strategy and prey importance: a new graphical analysis. J Fish Biol 36:261–263
Daase M, Vik JO, Bagøien E, Stenseth NCh, Eiane K (2007) The influence of advection on Calanus near Svalbard: statistical relations between salinity, temperature and copepod abundance. J Plankton Res 29:903–911
Daase M, Falk-Petersen S, Varpe Ø, Darnis G, Søreide JE, Wold A, Leu E, Berge J, Philippe B, Fortier L (2013) Timing of reproductive events in the marine copepod Calanus glacialis: a pan-Arctic perspective. Can J Fish Aquat Sci 70:871–884
Fort J, Cherel Y, Harding AMA, Welcker J, Jakubas D, Steen H, Karnovsky N, Grémillet D (2010) Geographic and seasonal variability in the isotopic niche of little auks. Mar Ecol Prog Ser 414:293–302
Gabrielsen GW, Taylor JRE, Konarzewski M, Mehlum M (1991) Field and laboratory metabolism and thermoregulation in dovekies (Alle alle). Auk 108:71–78
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Harding AMA, Pelt TIV, Lifjeld JT, Mehlum F (2004) Sex differences in little auk Alle alle parental care: transition from biparental to parental-only care. Ibis 146:642–651
Harding AMA, Egevang C, Walkusz W, Merkel F, Blanc S, Grémillet D (2009) Estimating prey capture rates of a planktivorous seabird, the little auk (Alle alle), using diet, diving behaviour, and energy consumption. Polar Biol 32:785–796
Harris R, Wiebe L, Lenz J, Skjoldal HR, Huntley M (2000) ICES zooplankton methodology manual. Academic Press, London, p 684
Hedd A, Ryder JL, Cowen LL, Bertram DF (2002) Inter-annual variation in the diet, provisioning and growth of Cassin’s auklets at Triangle Island, British Columbia: responses to variation in ocean climate. Mar Ecol Prog Ser 229:221–232
Hipfner JM (2008) Matches and mismatches: ocean climate, prey phenology and breeding success in a zooplanktivorous seabird. Mar Ecol Prog Ser 368:295–304
Hipfner JM (2009) Euphausiids in the diet of a North Pacific seabird: annual and seasonal variation and the role of ocean climate. Mar Ecol Prog Ser 390:277–289
Hop H, Falk-Petersen S, Svendsen H, Kwasniewski S, Pavlov V, Pavlova O, Søreide JE (2006) Physical and biological characteristics of the pelagic system across Fram Strait to Kongsfjorden. Prog Oceanogr 71:182–231
Hovinen JE, Welcker J, Rabindranath A, Brown ZW, Hop H, Berge J, Steen H (2014a) At-sea distribution of foraging little auks relative to physical factors and food supply. Mar Ecol Prog Ser 503:263–277
Hovinen JEH, Wojczulanis-Jakubas K, Jakubas D, Hop H, Berge J, Kidawa D, Karnovsky NJ, Steen H (2014b) Fledging success of little auks in the high Arctic: Do provisioning rates and the quality of foraging grounds matter? Polar Biol 37:665–674
IPCC (2007) Climate change 2007: the physical science basis. Summary for policy makers. Contribution of Working Group I to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change. Cambridge University Press, Cambridge
Isaksen K (1995) The breeding population of little auk (Alle alle) in colonies in Hornsund and northwestern Spitsbergen. In: Isaksen K, Bakken V (eds) Seabird population in the northern Barents Sea. Meddelelser nr 135. Norsk PolarInstitutt, Oslo, pp 49–57
Jakubas D, Manikowska B (2011) The response of Grey Herons Ardea cinerea to changes in prey abundance. Bird Study 58:487–494
Jakubas D, Wojczulanis-Jakubas K (2011) Subcolony variation in phenology and breeding parameters in little auk Alle alle. Polar Biol 34:31–39
Jakubas D, Wojczulanis-Jakubas K, Walkusz W (2007) Response of Dovekie to changes in food availability. Waterbirds 30:421–428
Jakubas D, Głuchowska M, Wojczulanis-Jakubas K, Karnovsky NJ, Keslinka L, Kidawa D, Walkusz W, Boehnke R, Cisek M, Kwaśniewski S, Stempniewicz L (2011) Foraging effort does not influence body condition and stress level in little auks. Mar Ecol Prog Ser 432:277–290
Jakubas D, Iliszko L, Wojczulanis-Jakubas K, Stempniewicz L (2012) Foraging by little auks in the distant marginal sea ice zone during the chick-rearing period. Polar Biol 35:73–81
Jakubas D, Trudnowska E, Wojczulanis-Jakubas K, Iliszko L, Kidawa D, Darecki M, Błachowiak-Samołyk K, Stempniewicz L (2013) Foraging closer to the colony leads to faster growth in little auks. Mar Ecol Prog Ser 489:263–278
Jakubas D, Wojczulanis-Jakubas K, Iliszko L, Darecki M, Stempniewicz L (2014) Foraging strategy of the little auk Alle alle throughout breeding season–switch from unimodal to bimodal pattern. J Avian Biol 45:551–560
Jakubas D, Iliszko LM, Strøm H, Darecki M, Jerstad K, Stempniewicz L (2016) Foraging behavior of a high-Arctic zooplanktivorous alcid, the little auk, at the southern edge of its breeding range. J Exp Mar Biol Ecol 475:89–99
Ji R, Jin M, Varpe Ø (2013) Sea ice phenology and timing of primary production pulses in the Arctic Ocean. Glob Change Biol 19:734–741
Joiris CR, Falck E (2011) Summer at-sea distribution of little auks Alle alle and harp seals Pagophilus (Phoca) groenlandica in the Fram Strait and the Greenland Sea: impact of small-scale hydrological events. Polar Biol 34:541–548
Karnovsky NJ, Kwasniewski S, Weslawski JM, Walkusz W, Beszczynska-Möller A (2003) Foraging behavior of little auks in a heterogeneous environment. Mar Ecol Prog Ser 253:289–303
Karnovsky NJ, Brown ZW, Welcker J, Harding AMA, Walkusz W, Cavalcanti A, Hardin J, Kitaysky A, Gabrielsen G, Grémillet D (2011) Inter-colony comparison of diving behavior of an Arctic top predator: implications for warming in the Greenland Sea. Mar Ecol Prog Ser 440:229–240
Kidawa D, Jakubas D, Wojczulanis-Jakubas K, Stempniewicz L, Trudnowska E, Keslinka-Nawrot L, Błachowiak-Samołyk K (2015) Parental efforts of an Arctic seabird, the little auk Alle alle, under variable foraging conditions. Mar Biol Res 11:349–360
Konarzewski M, Taylor JRE, Gabrielsen GW (1993) Chick energy requirements and adult energy expenditures of Dovekies (Alle alle). Auk 110:603–609
Kwasniewski S, Gluchowska M, Jakubas D, Wojczulanis-Jakubas K, Walkusz W, Karnovsky N, Blachowiak-Samolyk K, Cisek M, Stempniewicz L (2010) The impact of different hydrographic conditions and zooplankton communities on provisioning little auks along the west coast of Spitsbergen. Prog Oceanogr 87:72–82
Kwasniewski S, Gluchowska M, Walkusz W, Karnovsky NJ, Jakubas D, Wojczulanis-Jakubas K, Harding AMA, Goszczko I, Cisek M, Beszczynska-Moller A, Walczowski W, Weslawski JM, Stempniewicz L (2012) Interannual changes in zooplankton on the West Spitsbergen Shelf in relation to hydrography and their consequences for the diet of planktivorous seabirds. ICES J Mar Sci 69:890–901
Leu E, Søreide JE, Hessen DO, Falk-Petersen S, Berge J (2011) Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas: timing, quantity, and quality. Prog Oceanogr 90:18–32
Loeng H, Drinkwater K (2007) An overview of the ecosystems of the Barents and Norwegian Seas and their response to climate variability. Deep-Sea Res II 54:2478–2500
Lønne OJ, Gulliksen B (1991) On the distribution of sympagic macro-fauna in the seasonally ice covered Barents Sea. Polar Biol 11:457–469
Morrison ML, Ralph CJ, Verner J, Jehl JR Jr (1990) Avian foraging: theory, methodology, and applications. Stud Avian Biol 13:1–515
Murdoch WW, Avery S, Smyth MEB (1975) Switching in predatory fish. Ecology 56:1094–1105
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Piechura J, Beszczynska-Möller A, Osinski R (2001) Volume, heat and salt transport by the West Spitsbergen Current. Polar Res 20:233–240
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Saloranta T, Svendsen H (2001) Across the Arctic front west of Spitsbergen: high resolution CTD sections from 1998 to 2000. Polar Res 20:177–184
Scott CL, Falk-Petersen S, Sargent JR, Hop H, Lonne OJ, Poltermann M (1999) Lipids and trophic interactions of ice fauna and pelagic zooplankton in the marginal ice zone of Barents Sea. Polar Biol 21:65–71
Shultz MT, Piatt JF, Harding AM, Kettle AB, Van Pelt TI (2009) Timing of breeding and reproductive performance in murres and kittiwakes reflect mismatched seasonal prey dynamics. Mar Ecol Prog Ser 393:247–258
Søreide JE, Leu E, Berge J, Graeve M, Falk-Petersen S (2010) Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob Change Biol 16:3154–3163
Stempniewicz L (1980) Factors influencing the growth of the Little Auk, Plautus alle (L.), nestlings on Spitsbergen. Ekol Pol 28:557–581
Stempniewicz L (2001) Alle alle little auk. BWP update. J Birds West Palearctic 3:175–201
Stempniewicz L, Darecki M, Trudnowska E, Blachowiak-Samolyk K, Boehnke R, Jakubas D, Keslinka-Nawrot L, Kidawa D, Sagan S, Wojczulanis-Jakubas K (2013) Visual prey availability and distribution of foraging little auks (Alle alle) in the shelf waters of West Spitsbergen. Polar Biol 36:949–955
Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan KS, Lima M (2002) Ecological effects of climate fluctuations. Science 297:1292–1296
Svensson E (1995) Avian reproductive timing: When should parents be prudent? Anim Behav 49:1569–1575
Sydeman WJ, Hester MM, Thayer JA, Gress F, Martin P, Buffa J (2001) Climate change, reproductive performance and diet composition of marine birds in the southern California Current system, 1969–1997. Prog Oceanogr 49:309–329
Taguchi Y-H, Oono Y (2005) Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics 21:730–740
Taylor JRE, Konarzewski M (1989) On the importance of fat reserves for the little auk (Alle alle) chicks. Oecologia 81:551–558
Tranter DJ, Fraser H (1968) Zooplankton sampling. Monographs on Oceanographic Methodology 2. UNESCO, Paris
Trudnowska E, Szczucka J, Hoppe L, Boehnke R, Hop H, Błachowiak-Samołyk K (2012) Multidimensional zooplankton observations on the northern West Spitsbergen Shelf. J Mar Syst 98–99:18–25
Trudnowska E, Sagan S, Kwasniewski S, Darecki M, Blachowiak-Samolyk K (2015) Fine-scale zooplankton vertical distribution in relation to hydrographic and optical characteristics of the surface waters on the Arctic shelf. J Plankton Res 37:120–133
Walczowski W, Piechura J (2007) Pathways of the Greenland Sea warming. Geophys Res Lett 34:L10608. doi:10.1029/2007GL029974
Walczowski W, Piechura J, Osinski R, Wieczorek P (2005) The West Spitsbergen current volume and heat transport from synoptic observations in summer. Deep-Sea Res I 52:1374–1391
Watanuki Y, Kato A, Mori Y, Naito Y (1993) Diving performance of Adélie penguins in relation to food availability in fast sea-ice areas: comparison between years. J Anim Ecol 62:634–646
Weslawski JM, Stempniewicz L, Mehlum F, Kwasniewski S (1999) Summer feeding strategy of the little auk Alle alle from Bjørnøya, Barents Sea. Polar Biol 21:129–134
Wojczulanis-Jakubas K (2007) Male and female parental investments in the monogamous seabird (little auk Alle alle) in the conditions of the midnight sun. Unpublished PhD thesis (in Polish). University of Gdańsk, Gdańsk
Wojczulanis-Jakubas K, Jakubas D (2012) When and why does my mother leave me? The question of brood desertion in the Dovekie (Alle alle). Auk 129:632–637
Zach R (1988) Growth-curve analysis: a critical reevaluation. Auk 105:208–210
Acknowledgments
The study was supported by grant from Norway through the Norwegian Financial Mechanism (ALKEKONGE, PNRF-234-AI-1/07). We are indebted to the captain and crew of the R/V Oceania (IOPAS) and Lance (NPI) for their assistance during ALKEKONGE project cruises. We thank Lech Iliszko, Mateusz Barcikowski, Magdalena Hadwiczak, Liliana Keslinka and Adam Nawrot for assistance in the field. We would like to acknowledge Haakon Hop (NPI) assistance dealing with the second sampling of Magdalenefjorden. We appreciate the improvements of English usage made by Peter Senn. Data collection was performed under permits from the Governor of Svalbard and Norwegian Animal Research Authority.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jakubas, D., Wojczulanis-Jakubas, K., Boehnke, R. et al. Intra-seasonal variation in zooplankton availability, chick diet and breeding performance of a high Arctic planktivorous seabird. Polar Biol 39, 1547–1561 (2016). https://doi.org/10.1007/s00300-015-1880-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1880-z