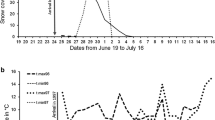
Abstract
Demographic parameters were estimated for snow petrels Pagodroma nivea nesting at the study colony of Reeve Hill near Casey station, Antarctica between 1984 and 2003. Average breeding success for the colony varied from 18.2% to 76.5%. Breeding effort, hatching and fledging success were subject to a high interannual variability. We examined the influence of regional sea-ice extent on the breeding performance of snow petrels at Reeve Hill. Fewer birds were breeding when sea-ice had been extensive during April–May. Overall breeding success and fledging success were improved during years with extensive sea-ice cover in winter. Successful breeding effort and breeding success were depressed when there was extensive sea-ice cover during January–February. Sea surface temperatures also correlated to snow petrel breeding performance parameters. Previous work showed that large-scale climatic events (ENSO, Antarctic circumpolar wave) and the related sea-ice cover around the Antarctic might affect the lower trophic levels of the marine environment and consequently food availability for snow petrels. A comparison with the long-term study conducted at Ile des Pétrels (Terre Adélie) suggests that despite similarities in the underlying biological processes that control snow petrel breeding performance, the nature of the correlation of large-scale environmental factors with breeding performance differs substantially between the two colonies, probably because of the confounding effects of other environmental factors acting at a local scale (local weather, nest quality), which also affect bird body condition.




Similar content being viewed by others
References
Ainley DG, O’Connor EF, Boekelheide RJ (1984) The marine ecology of seabirds in the Ross Sea, Antarctica. Ornith Monog 32:1–97
Ainley DG, Fraser WR, Sulllivan CW, Jones JJ, Hopkins TL, Smith WO (1986) Antarctic mesopelagic micronecton: evidence from seabirds that pack ice affects community structure. Science 232:847–849
Ainley DG, Boekelheid, RJ (1990) Seabirds of the Farallon islands: ecology, structure and dynamics of an upwelling-system community. Standford University Press, Stanford, California
Ainley DG, Ribic CA, Spear LB (1993) Species-habitat relationships among Antarctic seabirds: a function of physical or biological factors? Condor 95:806–816
Barbraud C (1999) Subspecies-selective predation of snow petrels by skuas. Oikos 86:275–282
Barbraud C, Chastel O (1999) Early body condition and hatching success in the snow petrel Pagodroma nivea. Polar Biol 21:1–4
Barbraud C, Weimerskirch H (2001) Contrasting effects of the extent of sea-ice on the breeding performance of an Antarctic top predator, the snow petrel Pagodroma nivea. J Avian Biol 32:297–302
Barbraud C, Weimerskirch H, Guinet C, Jouventin P (2000) Effect of sea-ice extent on adult survival of an Antarctic top predator: the snow petrel Pagodroma nivea. Oecologia 125:483–488
Büßer C, Kahles A,Quillfelt P (2004) Breeding success and chick provisioning in Wilson’s storm petrels Oceanites oceanicus over seven years: frequent failures due to food shortage and entombment. Polar Biol 27:613–622
Chastel O, Weimerskirch H, Jouventin P (1993) High annual variability in reproductive success and survival of an Antarctic seabird, the snow petrel Pagrodoma nivea. Oecologia 94:278–285
Cline DR, Siniff DB, Erickson AW (1969) Summer birds of the pack ice in the Weddell Sea, Antarctica. Auk 86:701–716
Coulson JC (1968) Differences in the quality of birds nesting in the center and on the edges of the colony. Nature 217:478–479
Craig PC, Griffiths WB, Haldorson L, McElderry H (1982) Ecological studies on Arctic cod Boreogadus saida in Beaufort coastal, Alaska. Can Tech Rep Fish Aquat Sci 39:395–406
Croxall JP (1992) Southern Ocean environmental changes: effects on seabirds, seals and whales populations. Philos Trans R Soc Lond B 338:319–328
Croxall JP, North AW (1988) Fish prey of Wilson’s storm petrel Oceanites oceanicus at South Georgia. BAS Bull 78:37–42
Croxall JP, Steele WK, McInnes SJ, Prince P (1995) Breeding distribution of the snow petrel Pagodroma nivea. Mar Ornithol 23:69–99
Croxall JP, Trathan PN, Murphy EJ (2002) Environmental change and Antarctic seabird populations. Science 297:1510–1514
Duffy DC (1983) Competition for nesting space among peruvian guano birds. Auk 100:680–688
Erikstad KE, Fauchald P, Tveraa T, Steen H (1998) On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79:1781–1788
van Franeker JA (1991) Casey snow petrel monitoring project. Manual. 24 pages. Unpublished report
van Franeker JA (2001). Mirrors in ice. Fulmarine petrels and Antarctic ecosystems. PhD Thesis. University of Groningen, p 274
van Franeker JA, Creuwels JCS, Van der Veer W, Cleland S, Robertson G (2001a). Unexpected effects of climate change on the predation of Antarctic petrels. Antarct Sci 13:430–439
van Franeker JA, Williams R, Imber MJ, Wolff WJ (2001b) Diet and foraging ecology of southern fulmar (Fulmarus glacialoides), Antarctic petrel Thalassoica antarctica, cape petrel Daption capense and snow petrels Pagodroma nivea ssp on Ardery Island, Wilkes Land, Antarctica. Chapter 11 (p 58) In: JA van Franeker, Mirrors in ice. PhD Thesis, University of Groningen. Alterra, Texel
Fraser WR, Trivelpiece WZ, Ainley DG, Trivelpiece SG (1992). Increases in Antarctic penguin populations: reduced competition with whales or a loss of sea-ice due to environmental warming? Polar Biol 11:525–531
Gleason JR (1988) Algorithms for balanced bootstrap simulations. Am Statist 42:263–266
Griffiths AM (1983) Factors affecting the distribution of snow petrel Pagodroma nivea and the Antarctic petrel Thalassoica antarctica. Ardea 71:145–150
Guillotin M, Jouventin P (1980) Le Pétrel des Neiges a Pointe Géologie. Le Gerfaut 70:51–72
Guinet C, Jouventin P, Georges JY (1994) Long term population changes of fur seals Arctocephalus gazella and A. tropicalis on subantarctic (Crozet) and Subtropical (St Paul and Amsterdam Islands) and their possible relationship to El Niño Southern Oscillation. Antarct Sci 6:473–478
Guinet C, Chastel O, Koudil M, Durbec JP, Jouventin P (1998) Effects of warm sea surface temperature anomalies on the blue petrel at the Kerguelen islands. Proc R Soc Lond B 265:1001–1006
Haddon M. (2001) Modelling and quantitative methods in fisheries. CRC/Chapman and Hall, London
Hall P (1992) The bootstrap and edgeworth expannon. Springer Verlag, Berlin, Heidelberg, New York
Hudson R (1966) Adult survival estimates for two Antarctic Petrels. BAS Bull 8:63–73
Hunt GL Jr, Priddle J, Whitehouse MJ, Veit RR, Heywood RB (1992) Changes in seabird species abundance near South Georgia during a period of rapid change in sea surface temperature. Antarct Sci 4:15–22
Jackson GD, Domeier ML (2003) The effects of an extraordinary El Niño/La Niña event on the size and growth of the squid Loligo opalescens off Southern California. Mar Biol 142:925–935
Jouventin P, Bried J (2001) The effect of mate choice on speciation in snow petrels. Anim Behav 62:123–132
Jouventin P, Weimerskirch H (1991) Changes in the population size and demography of southern seabirds: management implications. In: Lebreton JD, Hirons, GJM (eds) Bird population studies: relevance to conservation and management. Oxford University Press, Oxford, pp 297–314
Loeb, V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997). Effects of sea-ice extent on krill and salp dominance on the Antarctic food web. Nature 387:897–900
Marchant S, Higgins PJ (1990) Handbook of Australian, New Zealand and Antarctic birds. Oxford University Press, Melbourne
Micol T, Jouventin P (2001) Long-term population trends in seven Antarctic seabirds at Pointe Géologie (Terre Adélie): human impact compared with environmental change. Polar Biol 24:175–185
Montague TL (1988) Birds of Prydz bay, Antarctica—distribution and abundance. Hydrobiologia 165:227–237
Reynolds RW, Smith TM (1995) A high resolution global sea-surface temperature climatology. J Clim 8(6):1571–1583
Ridoux V, Offredo C (1989) The diets of five summer breeding seabirds in Adélie Land, Antarctica. Polar Biol 9:137–145
Saether B-E, Lorentsen S-H, Tverra T, Andersen R, Pedersen HC (1997). Size dependent variation in reproductive success of an Antarctic seabird, the Antarctic petrel Thalassoica antarctica. Auk 114:333–340
Siegel V, Loeb V (1995) Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar Ecol Prog Ser 123:45–56
Smith WO, Nelson DM (1986) Importance of ice-edge phytoplankton production in the Southern ocean. Bioscience 36:251–257
Steele WK (1994) Snow petrel Pagodroma nivea chick development at an inland colony in Queen Maud Land, Antarctica. In: Antarctic communities, species, structure and survival. Abstracts SCAR sixth biology symposium. Venice, May 1994, p 254
Sullivan C, Arrigo KR, McClain CR, Comiso JC, Firestone J (1993) Distributions of phytoplankton blooms in the Southern ocean. Science 262:1832–1837
Trathan PN, Croxall JP, Murphy EJ (1996) Dynamics of Antarctic penguin populations in relation to interannual variability in sea-ice distribution. Polar Biol 16:321–330
Tveraa T, Christensen GN (2002) Body condition and parental decisions in the snow petrel Pagodroma nivea. Auk 119:266–270
Veit RR, Hunt GL (1991) Broadscale density and aggregation of pelagic birds from a circumnavigational survey of the Antarctic ocean. Auk 108:790–800
Weathers WW, Gerhart KL, Hodum PJ (2000) Thermoregulation in Antarctic fulmarine petrels. J Comp Physiol B Biochem Syst Environ Physiol 170:561–572
Weimerskirch H, Inchausti P,Guinet C, Barbraud C (2003) Trends in birds and seal populations as indicators of a system shift in the Southern Ocean. Antarct Sci 15(2):249–256
White WB, Peterson RG (1996) An Antarctic circumpolar wave in surface pressure, wind, temperature and sea-ice extent. Nature 380:699–702
White WB, Chen S-C, Allan RJ, Stone RC (2002) Positive feedbacks between the Antarctic Circum polar wave and the global El Nino-Southern Oscillation wave. J Geophys Res 107:3165–3182
Whitehead MD, Johnstone GW, Burton HR (1990) Annual fluctuations in productivity and breeding success of Adélie penguins and fulmarine petrels in Prydz Bay, East Antarctica. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, Heidelberg, New York, pp 214–223
Wilson PR, Ainley DG, Nur N, Jacobs SS, Barton KJ, Ballard G, Comiso JC (2001). Adélie penguin population change in the pacific sector of Antarctica: relation to sea-ice extent and the Antarctic circumpolar current. Mar Ecol Prog Ser 213:301–309
Wolter K, Timlin MS (1998) Measuring the strength of ENSO—how does 1997/98 rank? Weather 53:315–324
Acknowledgements
The authors are grateful to L. Belbin, G. Jackson and B. Raymond for their comments on an early draft. B. Raymond also provided very valuable help for sea-ice data processing and Dr S. Wotherspoon helped with some of the statistical analysis of the data. We thank three anonymous reviewers, who kindly suggested improvements to the original manuscript. We are grateful to A. Beinssen, P. Bell, A. Breed, G. Browning, E. Bulling, S. Creet, C. Deacon, T. Donohue, R. Givney, A. Hackett, O. Hentschel, M. Hovenden, A. Jackson, A. Lee, T. Maddern, B. Matheson, T. Montague, P. Nicholls, M. Pertout, S. Pearce, A. Post, J. Reynolds, J. Stark, R. Seppelt, D. Simon, A. Watson, R. Watzl, G. Browning and many unnamed Casey personnel for their participation in the Reeve Hill long-term monitoring program. This project is now part of an ongoing program on long-term monitoring of bird populations supported by the Antarctic Science Advisory Committee (ASAC project 1219).
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Methods used for the comparison of breeding parameters obtained for a constant subset of nests and a varying number of nests at Reeve Hill between 1984 and 2002.
Logistic regression was used for four of the five reproductive performance parameters studied. Data were separated into two groups: group A, the subset of 42 nests and group B, all the remaining nests (this number varied from year to year). A logistic regression model of the form logit(pi_ij)=M + Year_i + Group_j was fitted. With this model, M was a constant, Year was treated as a factor (not as a covariate), and the significance of the term group (A or B) was tested. Analyses were conducted for breeding effort, hatching success, fledging success and breeding success. The four analyses compared the fit of the model that allowed the proportion of success to vary between years and between groups to the fit of the model that only allowed the proportion of success to vary between years. Group A and B did not differ significantly in breeding effort, hatching success, or breeding success (P>0.1 for each model). For fledging success, the dataset on which the fit of the models was tested was greatly reduced due to the presence of null values (0 fledging against 0 egg hatched in group B). The difference between groups was significant (P=0.020), but is not clear which years were associated with group differences and the reduced number of data points (only nine) limits conclusions. Overall, we are confident that breeding performance parameters obtained for a subset of nests consistently checked were not significantly different from those obtained for the entire study colony (with a varying number of nests). Thus, in order to remain consistent with the study at Ile des Pétrels, all subsequent analyses and results presented, in this paper, were conducted with breeding performance parameters calculated for the entire study colony.
Rights and permissions
About this article
Cite this article
Olivier, F., Franeker, J.A.v., Creuwels, J.C.S. et al. Variations of snow petrel breeding success in relation to sea-ice extent: detecting local response to large-scale processes?. Polar Biol 28, 687–699 (2005). https://doi.org/10.1007/s00300-005-0734-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-005-0734-5