Abstract
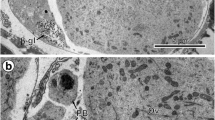
Antarctic teleosts perform their physiological activities at constant subzero temperatures. We previously described the gross morphology and the biochemical composition of the Antarctic teleost Chionodraco hamatus eggshell. In this work, we investigate thoroughly the chorion ultrastructure, showing a previously unknown external layer, and describe the preparation and use of monoclonal antibodies against eggshell proteins of this species. The main chorion polypeptide at 46 kDa was purified by preparative electrophoresis and used as immunogen in mice. After spleen-myeloma fusion, hybdridomas were screened by immunoblotting against eggshell homogenates, and two of the most interesting hybridomas were cloned by limiting dilution, and established in culture: CHE-1 (IgG) and CHE-5 (IgM). They stained intensely the eggshell in indirect immunofluorescence and in immunoelectron microscopy. The CHE-5 localisation on thin sections by immunogold staining was peculiar for the various vitelline envelope layers. By western blot analysis of eggshell, CHE-1 recognised two polypeptides at 46 kDa and 92 kDa, whereas CHE-5 recognised a single polypeptide at 92 kDa.




Similar content being viewed by others
References
Baldacci A, Taddei AR, Mazzini M, Fausto AM, Buonocore F, Scapigliati G (2001) Ultrastructure and proteins of the egg chorion of the antarctic fish Chionodraco hamatus (Teleostei, Notothenioidei). Polar Biol 24:417–421
Begovac PC, Wallace RA (1989) Major vitelline envelope proteins in pipefish oocytes originate within the follicle and are associated with the Z3 layer. J Exp Zool 251:56–73
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brivio MF, Bassi R, Cotelli F (1991) Identification and characterization of the major components of the Oncorhynchus mykiss egg chorion. Mol Reprod Dev 28:85–93
Chang YS, Lu LF, Lai CY, Kou YH, Huang FL (1999) Purification, characterization and molecular cloning of an outer layer protein of carp fertilization envelope. Mol Reprod Dev 54:186–193
Del Giacco L, Vanoni C, Bonsignorio D, Duga S, Mosconi G, Santucci A, Cotelli F (1998) Identification and spatial distribution of the mRNA encoding the gp49 component of the gilthead sea bream, Sparus aurata egg envelope. Mol Reprod Dev 49:58–69
Del Giacco L, Diani S, Cotelli F (2000) Identification and spatial distribution of the mRNA encoding an egg envelope component of the Cyprinid zebrafish, Danio rerio, homologous to the mammalian ZP3 (ZPC). Dev Genes Evol 210:41–46
Dumont JN, Brummett AR (1985) Egg envelopes in vertebrates. In: Browder RW (ed) Developmental biology, vol 2. Plenum, New York, pp 235–288
Eastman JT, DeVries AL (1987) I pesci dell’Antartide. Le Scienze 22:68–75
Fausto AM, Carcupino M, Scapigliati G, Taddei AR, Mazzini M (1994) Fine structure of the chorion and micropyle of the sea bass egg Dicentrarchus labrax (Teleostea, Percichthydae). Boll Zool 61:129–133
Ha CR, Iuchi I (1998a) Enzyme responsible for egg envelope (chorion) hardening in fish: purification and partial characterization of two transglutaminases associated with their substrate, fertilized egg chorion, of the rainbow trout, Oncorhynchus mykiss. J. Biochem Tokyo 124:917–926
Ha CR, Iuchi I (1998b) Extraction and partial characterization of egg envelope (chorion) transglutaminase of rainbow trout Oncorhynchus mykiss: properties for efficient chorion hardening. Comp Biochem Physiol B 118:293–301
Hamazaki T, Iuchi I, Yamagami K (1985) A spawning female-specific substance reactive to anti-chorion (egg envelope) glicoprotein antibody in the teleost Oryzias latipes. J Exp Zool 235:269–279
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Spring Harbor, New York
Hubold G, Ekau W (1990) Feeding patterns of post-larval and juvenile Notothenioids in the Southern Weddell sea (Antarctica). Polar Biol 10:255–260
Hyllner SJ, Haux C (1992) Immunochemical detection of the major vitelline envelope proteins in the plasma and oocytes of the maturing female rainbow trout, Oncorhynchus mykiss. J Endocrinol 135:303–309
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137a–138a
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. A review. Antarct Sci 3:125–150
Ludwig H (1874) Gber die eibildung thierreiche. Arb Zool Zoot Inst Wurzburg 1:287–510
Lyons CE, Payette KL, Price JL, Huang RCC (1993) Expression and structural analysis of a teleost homolog of mammalian zona pellucida gene. J Biol Chem 268:21351–21358
Murata K, Iuchi I, Yamagami K (1994) Synchronous production of the low- and high-molecular weight precursors of the egg envelope subunits, in response to estrogen administration in the teleost fish Oryzias latipes. Gen Comp Endocrinol 95:232–239
Murata K, Sugiyama H, Yasumasu S, Iuchi I, Yasumasu I, Yamagami K (1997) Cloning of cDNA and estrogen-induced hepatic gene expression for choriogenin H, a precursor of the fish egg envelope (chorion). Proc Natl Acad Sci U S A 94:2050–2055
Oppen-Berntsen DO, Helvik JV, Walther BT (1990) The major structural protein of cod (Gadus morhua) eggshells and protein crosslinking during teleost egg hardening. Dev Biol 137:258–265
Riehl R (1993) Surface morphology and micropyle as a tool for identifying fish eggs by scanning electron microscopy. Eur Microsc Anal 7–9
Riehl R, Ekau W (1990) Identification of Antarctic fish eggs by surface structure as shown by the eggs of Trematomus eulepidotus (Teleostei: Notothenioidae). Polar Biol 11:27–31
Riehl R, Kock KH (1989) The surface structure of antarctic fish eggs and its use in identifying fish eggs from the southern ocean. Polar Biol 9:197–203
Scapigliati G, Carcupino M, Mazzini M (1992) Effect of freshwater on unfertilized eggs of Oncorhynchus mykiss (Walbaum) (Pisces, Teleostea): a preliminary electrophoretic and scanning electron microscopy study of the eggshell. Anim Biol 2:45–49
Scapigliati G, Carcupino M, Taddei AR, Mazzini M (1994) Characterization of the main egg envelope proteins of the sea bass Dicentrarchus labrax. Mol Reprod Dev 38:48–53
Scapigliati G, Mazzini M, Mastrolia L, Romano N, Abelli L (1995) Production and characterization of a monoclonal antibody against the thymocytes of the sea bass Dicentrarchus labrax (L.) (Teleostea, Percichthydae). Fish Shellfish Immunol 5:393–405
Scapigliati G, Meloni S, Mazzini M (1999) A monoclonal antibody against chorion proteins of the sea bass Dicentrarchus labrax (L.): studies of chorion precursors and applicability in immunoassays. Biol Reprod 60:783–789
Shandikov GA, Faleeva TI (1992) Features of gametogenesis and sexual cycles of six notothenioid fishes from East Antarctica. Polar Biol 11:615–621
Vacchi M, Williams R, La Mesa M (1996) Reproduction in three species of fish from the Ross Sea and Mawson Sea. Antarct Sci 8:185–192
Yamagami K, Hamazaki TS, Yasumasu S, Masuda K, Iuchi I (1992) Molecular and cellular basis of formation, hardening, and breakdown of the egg envelope in fish. Int Rev Cytol 136:51–92
Acknowledgements
The authors are grateful to the Italian Program of Antarctic Research (PNRA), which supported the research and allowed fish collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meloni, S., Mazzini, M., Fausto, A.M. et al. Egg envelope organisation in the icefish Chionodraco hamatus . Polar Biol 27, 586–594 (2004). https://doi.org/10.1007/s00300-004-0631-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-004-0631-3