Abstract
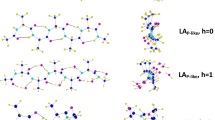
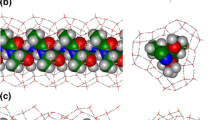
The structure and association energy of hydrated l-alanine pentamers at the hydration rate (h) of 0–1 were calculated by quantum chemical calculation (QCC) using the density functional theory [B3LYP/6-31G(d, p)] method for three kinds of stable conformers (β-extended: t−/t+, PPII-like: g−/g+, α-helix: g−/g−) converged by convergent calculations from l-alanine pentamers in gas phase. Water molecules are mainly inserted between intramolecular hydrogen bond of CO–HN in PPII-like and α-helix conformers and attached to the CO group in β-extended conformer. α-Helix conformer turns to PPII-like conformer at higher hydration rate. The association energy decreased with the increase of the hydration rate, indicating that the conformers were stabilized by the hydration. It was found that PPII-like conformer, which was the most stable in the anhydrate state, was also the most stable in the hydrate state. This structure corresponds to a middle structure of PPII (g−/t+) and β-extended (t−/t+) structures. These results obtained by energy calculation using QCC support the experimental results by Eker et al. and Graf et al., who reported that alanine oligomer exhibited a mixture of PPII and β-extended structure in aqueous solution.




Similar content being viewed by others
References
Marqusee S, Robbins VH, Baldwin RL (1989) Unusually stable helix formation in short alanine-based peptides. Proc Natl Acad Sci USA 86:5286
Eker F, Cao X, Nafie L, Schweitzer-Stenner R (2002) Tripeptides adopt stable structures in water. A combined polarized visible raman, FTIR, and VCD spectroscopy study. J Am Chem Soc 124:14330
Eker F, Griebenow XK, Schweitzer-Stenner R (2003) Stable conformation of tripeptides in aqueous solution studied by UV circular dichroism spectroscopy. J Am Chem Soc 125:8178
Shi Z, Oison CA, Rose GD, Baldwin RL, Kallenbach NR (2002) Polyproline II structure in a sequence of seven alanine residues. PNAS 99:9190
Graf J, Nguyen PH, Stock G, Schwalbe H (2007) Structure and dynamics of the homologous series of alanine peptides: a joint molecular dynamics/NMR study. J Am Chem Soc 129:1179
Mu Y, Kosov DS, Stock G (2003) Conformational dynamics of trialanine in water. 2. Comparison of AMBER, CHARMM, GROMOS, and OPLS force fields to NMR and infrared experiments. J Phys Chem B 107:5064
Kentsis A, Mezei M, Gindin T, Osman R (2004) Unfolded state of polyalanine is a segmented polyproline II helix. Proteins Struct Funct Bioinf 55:493
Bour P, Kubelka J, Keiderling TA (2002) Ab initio quantum mechanical models of peptide helices and their vibrational spectra. Biopolymers 65:45
Kobayashi M, Sim JH, Sato H (2015) Conformational analyses for alanine oligomer during chain propagation by quantum chemical calculation. Polymer J 47:369
Gaussian 03 User’s Reference (2003) Gaussian Inc., PA, USA
Rigaudy J, Klesney SP (1979) Nomenclature of Organic Chemistry, section E 483 Pergamon Press, Oxford
Kinoshita M, OkamotoY Hirata F (2000) Solvent effects on formation of tertiary structure of protein. Seibutsu-butsuri Biophys Soc Jpn 40:374
Kobayashi M, Takahashi M, Sato H (2009) Conformational analysis for hydrated ethylene oxide oligomer models by quantum chemical calculations. Polym Bull 63:299
Kobayashi M, Takahashi M, Sato H (2009) Conformational analysis for hydrated ethylene imine oligomer model by quantum chemical calculations. Polym J 41:880
Kobayashi M, Sato H (2010) Structure analysis for hydrate model of ethyleneimine oligomer by quantum chemical calculation. Pharmacol Pharm 1:60
Kobayashi M, Takahashi M, Sato H (2011) Estimations of conformational effects using hydrated poly(ethyleneimine) models by quantum chemical calculation. Kobunshi Ronbunshu 68:647
Ludwig R (2001) Water from cluster to the bulk. Angew Chem Int Ed 40:1808
Dyke TR, Mack KM, Muenter JS (1977) J Chem Phys 66:498
Odutola JA, Dyke TR (1980) J Chem Phys 72:5062
Choi J, Hahn S, Cho M (2006) Vibrational spectroscopic characteristics of secondary structure polypeptides in liquid water: constrained MD simulation studies. Biopolymers 83:519
Mirkin NG, Krimm S (2007) Conformation dependence of the CαDα stretch mode in peptides 1. Isolated alanine peptide structures. J Phys Chem A 111:5300
Foresman JB, Keith TA, Wiberg KB, Snoonian J, Frisch MJ (1996) J Phys Chem 100:16098
Lide DR (2001) CRC handbook of chemistry and physics. CRC Press, London, pp 8–127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, M., Sim, J.H. & Sato, H. Conformational analyses for alanine oligomer during hydration by quantum chemical calculation (QCC). Polym. Bull. 74, 657–670 (2017). https://doi.org/10.1007/s00289-016-1736-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1736-x