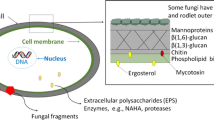
Abstract
Inhalation of fungal particles is a ubiquitous way of exposure to microorganisms during human life; however, this exposure may promote or exacerbate respiratory diseases only in particular exposure conditions and human genetic background. Depending on the fungal species and form, fungal particles can induce symptoms in the lung by acting as irritants, aeroallergens or pathogens causing infection. Some thermophilic species can even act in all these three ways (e.g. Aspergillus, Penicillium), mesophilic species being only involved in allergic and/or non-allergic airway diseases (e.g. Cladosporium, Alternaria, Fusarium). The goal of the present review is to present the current knowledge on the interaction between airborne fungal particles and the host immune system, to illustrate the differences of immune sensing of different fungal species and to emphasise the importance of conducting research on non-conventional mesophilic fungal species. Indeed, the diversity of fungal species we inhale and the complexity of their composition have a direct impact on fungal particle recognition and immune system decision to tolerate or respond to those particles, eventually leading to collateral damages promoting airway pathologies.
Similar content being viewed by others
References
Yamamoto N, Bibby K, Qian J, Hospodsky D, Rismani-Yazdi H et al (2012) Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J 6:1801–1811
Vesper SJ, Varma M, Wymer LJ, Dearborn DG, Sobolewski J et al (2004) Quantitative polymerase chain reaction analysis of fungi in dust from homes of infants who developed idiopathic pulmonary hemorrhaging. J Occup Environ Med 46:596–601
Selman M, Lacasse Y, Pardo A, Cormier Y (2010) Hypersensitivity pneumonitis caused by fungi. Proc Am Thorac Soc 7:229–236
Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L et al (2012) Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol 130:639–644, e5
Reponen T, Vesper S, Levin L, Johansson E, Ryan P et al (2011) High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann Allergy Asthma Immunol 107:120–126
Vesper S, Barnes C, Ciaccio CE, Johanns A, Kennedy K et al (2013) Higher environmental relative moldiness index (ERMI) values measured in homes of asthmatic children in Boston, Kansas City, and San Diego. J Asthma 50:155–161
Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM (2006) The link between fungi and severe asthma: a summary of the evidence. Eur Res J 27:615–626
Dannemiller KC, Mendell MJ, Macher JM, Kumagai K, Bradman A et al (2014) Next-generation DNA sequencing reveals that low fungal diversity in house dust is associated with childhood asthma development. Indoor Air 24:236–247
Linaker C, Smedley J (2002) Respiratory illness in agricultural workers. Occup Med (Lond) 52:451–459
Salvi SS, Barnes PJ (2009) Chronic obstructive pulmonary disease in non-smokers. Lancet 374:733–743
Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Environmental, Occupational Health Assembly ATS et al (2003) American thoracic society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 167:787–797
Mehta AJ, Miedinger D, Keidel D, Bettschart R, Bircher A et al (2012) Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the swiss cohort study on air pollution and lung and heart diseases in adults. Am J Respir Crit Care Med 185:1292–1300
Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA (2014) Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol 151:1–15
Knight DA, Holgate ST (2003) The airway epithelium: structural and functional properties in health and disease. Respirology 8:432–446
Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN et al (2012) IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 188:1503–1513
Walker JA, Barlow JL, McKenzie AN (2013) Innate lymphoid cells—how did we miss them? Nat Rev Immunol 13:75–87
Hammad H, Lambrecht BN (2007) Lung dendritic cell migration. Adv Immunol 93:265–278
Maldonado RA, von Andrian UH (2010) How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol 108:111–165
Romani L (2011) Immunity to fungal infections. Nat Rev Immunol 11:275–288
Bellanger AP, Pallandre JR, Borg C, Loeffert S, Gbaguidi-Haore H et al (2013) Human monocyte-derived dendritic cells exposed to microorganisms involved in hypersensitivity pneumonitis induce a Th1-polarized immune response. Clin Vaccine Immunol 20:1133–11142
Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R et al (2009) Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol 182:2502–2510
Zhu J, Paul WE (2008) CD4 T cells: fates, functions, and faults. Blood 112:1557–1569
Gaffen SL, Jain R, Garg AV, Cua DJ (2014) The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14:585–600
Liu Y, Zeng M, Liu Z (2014) Th17 response and its regulation in inflammatory upper airway diseases. Clin Exp Allergy. doi:10.1111/cea.12378
Li Q, Guo Z, Xu X, Xia S, Cao X (2008) Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol 38:2751–2761
van de Veerdonk FL, Kullberg BJ, van der Meer JW, Gow NA, Netea MG (2008) Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr Opin Microbiol 11:305–312
Levitz SM (2010) Innate recognition of fungal cell walls. PLoS Pathog 6:e1000758
Latge JP (2010) Tasting the fungal cell wall. Cell Microbiol 12:863–872
Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP (2009) Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121
Romani L (2004) Immunity to fungal infections. Nat Rev Immunol 4:1–23
Sancho D, Sousa R e C (2012) Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 30:491–529
Hoving JC, Wilson GJ, Brown GD (2014) Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol 16:185–194
Netea MG, Gow NA, Munro CA, Bates S, Collins C et al (2006) Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116:1642–1650
Hontelez S, Sanecka A, Netea MG, van Spriel AB, Adema GJ (2012) Molecular view on PRR cross-talk in antifungal immunity. Cell Microbiol 14:467–474
Hardison SE, Brown GD (2012) C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 13:817–822
Drummond RA, Brown GD (2013) Signalling C-type lectins in antimicrobial immunity. PLoS Pathog 9:e1003417
Goodridge HS, Wolf AJ, Underhill DM (2009) Beta-glucan recognition by the innate immune system. Immunol Rev 230:38–50
Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B et al (2009) Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol 10:203–213
LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC et al (2007) Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8:630–638
Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ et al (2014) CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med 211(11):2307-2321
Vautier S, Sousa Mda G, Brown GD (2010) C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev 21:405–412
Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M et al (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 13:246–254
Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N et al (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436
Mintz-Cole RA, Brandt EB, Bass SA, Gibson AM, Reponen T et al (2013) Surface availability of beta-glucans is critical determinant of host immune response to Cladosporium cladosporioides. J Allergy Clin Immunol 132(1):159-169
Mintz-Cole RA, Gibson AM, Bass SA, Budelsky AL, Reponen T et al (2012) Dectin-1 and IL-17A suppress murine asthma induced by Aspergillus versicolor but not Cladosporium cladosporioides due to differences in beta-glucan surface exposure. J Immunol 189:3609–3617
Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB et al (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361:1760–1767
Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C et al (2009) A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361:1727–1735
Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O et al (2013) Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr 25:736–747
Bueter CL, Specht CA, Levitz SM (2013) Innate sensing of chitin and chitosan. PLoS Pathog 9:e1003080
Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A et al (2008) Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4:e1000040
Fernandes C, Anjos J, Walker LA, Silva BM, Cortes L et al (2014) Modulation of Alternaria infectoria cell wall chitin and glucan synthesis by cell wall synthase inhibitors. Antimicrob Agents Chemother 58:2894–2904
Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B et al (2011) Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 73:479–501
Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N et al (2007) Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92–96
Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH et al (2011) Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J Biol Chem 286:35447–35455
Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG et al (2009) Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol 182:3573–3582
Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S et al (2014) Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog 10:e1004050
Ramaprakash H, Hogaboam CM (2010) Intranasal CpG therapy attenuated experimental fungal asthma in a TLR9-dependent and -independent manner. Int Arch Allergy Immunol 152:98–112
Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP et al (2013) C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39:324–334
Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P et al (2003) Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis 188:165–172
Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM (2001) Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol 166:4620–4626
van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC et al (2009) The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329–340
Bi L, Gojestani S, Wu W, Hsu YM, Zhu J et al (2010) CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem 285:25969–25977
Sato K, Yang XL, Yudate T, Chung JS, Wu J et al (2006) Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 281:38854–38866
Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Sousa R e C (2009) Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J ExpMed 206:2037–2051
Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H et al (2010) Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32:681–691
Cambi A, Gijzen K, de Vries IJ, Torensma R, Joosten B et al (2003) The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 33:532–538
Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y et al (2007) C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26:605–616
Serrano-Gomez D, Dominguez-Soto A, Ancochea J, Jimenez-Heffernan JA, Leal JA et al (2004) Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J Immunol 173:5635–5643
Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S et al (2008) The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180:7404–7413
Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E et al (2009) C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A 106:1897–1902
Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T et al (2013) Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 13:477–488
Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T et al (2014) Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe 15:494–505
Crameri R, Garbani M, Rhyner C, Huitema C (2014) Fungi: the neglected allergenic sources. Allergy 69:176–185
Wills-Karp M, Nathan A, Page K, Karp CL (2010) New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol 3:104–110
Kouzaki H, O'Grady SM, Lawrence CB, Kita H (2009) Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol 183:1427–1434
Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P (2000) Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 105:1185–1193
Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP et al (2013) Barrier disrupting effects of Alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One 8:e71278
Matsuwaki Y, Wada K, Moriyama H, Kita H (2011) Human eosinophil innate response to Alternaria fungus through protease-activated receptor-2. Int Arch Allergy Immunol 155(1):123–128
Ossovskaya VS, Bunnett NW (2004) Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84:579–621
Seltzer JM, Fedoruk MJ (2007) Health effects of mold in children. Pediatr Clin North Am 54:309–333, viii-ix
Wu F, Groopman JD, Pestka JJ (2014) Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol 5:351–372
Bondy GS, Pestka JJ (2000) Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev 3:109–143
Islam MR, Roh YS, Cho A, Kim J, Kim JH et al (2012) Immune modulatory effects of the foodborne contaminant citrinin in mice. Food Chem Toxicol 50:3537–3547
Schutze N, Lehmann I, Bonisch U, Simon JC, Polte T (2010) Exposure to mycotoxins increases the allergic immune response in a murine asthma model. Am J Respir Crit Care Med 181:1188–1199
Pestka JJ, Smolinski AT (2011) Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health, Part B 8:39–69
Wang YC, Deng JL, Xu SW, Peng X, Zuo ZC et al (2012) Effects of zearalenone on IL-2, IL-6, and IFN-gamma mRNA levels in the splenic lymphocytes of chickens. Sci World J 2012:567327
Pistol G, Gras M, Marin DE, Tabuc C, Taranu I (2013) Zearalenone induces alterations of hepatic immune responses by modulation of pro-inflammatory cytokines and matrix metalloproteinase gene expression. In: 6th International Workshop on Immunonutrition, PotN Society, Palma de Mallorca, p E2
Stoev SD, Gundasheva D, Zarkov I, Mircheva T, Zapryanova D et al (2012) Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp Toxicol Pathol 64:733–741
Pieckova E (2012) Adverse health effects of indoor moulds. Arh Hig Rada Toksikol 63:545–549
Korpi A, Jarnberg J, Pasanen AL (2009) Microbial volatile organic compounds. Crit Rev Toxicol 39:139–193
Matysik S, Herbarth O, Mueller A (2008) Determination of volatile metabolites originating from mould growth on wall paper and synthetic media. J Microbiol Methods 75:182–187
Inamdar AA, Bennett JW (2014) A common fungal volatile organic compound induces a nitric oxide mediated inflammatory response in Drosophila melanogaster. Sci Rep 4:3833
Hargreave FE, Nair P (2009) The definition and diagnosis of asthma. Clin Exp Allergy 39:1652–1658
Denning DW, Pashley C, Hartl D, Wardlaw A, Godet C et al (2014) Fungal allergy in asthma-state of the art and research needs. Clin Transl Allergy 4:14
Cai GH, Hashim JH, Hashim Z, Ali F, Bloom E et al (2011) Fungal DNA, allergens, mycotoxins and associations with asthmatic symptoms among pupils in schools from Johor Bahru, Malaysia. Pediatr Allergy Immunol 22:290–297
Zubairi AB, Azam I, Awan S, Zafar A, Imam AA (2014) Association of airborne Aspergillus with asthma exacerbation in Southern Pakistan. Asia Pacific allergy 4:91–98
Simon-Nobbe B, Denk U, Poll V, Rid R, Breitenbach M (2008) The spectrum of fungal allergy. Int Arch Allergy Immunol 145:58–86
Eduard W (2009) Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol 39:799–864
Hansel TT, Johnston SL, Openshaw PJ (2013) Microbes and mucosal immune responses in asthma. Lancet 381:861–873
Deckers J, Branco Madeira F, Hammad H (2013) Innate immune cells in asthma. Trends Immunol 34:540–547
Bartemes KR, Kephart GM, Fox SJ, Kita H (2014) Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 134:671–678, e4
Manni ML, Robinson KM, Alcorn JF (2014) A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respir Med 8:25–42
McAleer JP, Kolls JK (2014) Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev 260:129–144
Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF (2014) Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol 40:30–48
Agarwal R (2009) Allergic bronchopulmonary aspergillosis. Chest 135:805–826
Jat KR, Walia DK, Khairwa A (2013) Anti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev 9:CD010288
Knutsen AP (2011) Immunopathology and immunogenetics of allergic bronchopulmonary aspergillosis. J Allergy (Cairo) 2011:785983
Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W et al (2009) Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 119:1809–1818
Callejas CA, Douglas RG (2013) Fungal rhinosinusitis: what every allergist should know. Clin Exp Allergy 43:835–849
Hamilos DL (2014) Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol 133:640–653, e4
Glass D, Amedee RG (2011) Allergic fungal rhinosinusitis: a review. Ochsner J 11:271–275
Kern EB, Sherris D, Stergiou AM, Katz LM, Rosenblatt LC et al (2007) Diagnosis and treatment of chronic rhinosinusitis: focus on intranasal Amphotericin B. Ther Clin Risk Manag 3:319–325
Tang P, Mohan S, Sigler L, Witterick I, Summerbell R et al (2003) Allergic fungal sinusitis associated with Trichoderma longibrachiatum. J Clin Microbiol 41:5333–5336
Gupta AK, Shah N, Kameswaran N, Rai D, D.N. J, Chopra H et al (2012) Allergic fungal rhinosinusitis. Clin Rhinol: Int J 5:72–86
Cavanna C, Seminari E, Pusateri A, Mangione F, Lallitto F et al (2014) Allergic fungal rhinosinusitis due to Curvularia lunata. New Microbiol 37:241–245
Bakhshaee M, Fereidouni M, Mohajer MN, Majidi MR, Azad FJ et al (2013) The prevalence of allergic fungal rhinosinusitis in sinonasal polyposis. Eur Arch Otorhinolaryngol 270:3095–3098
Ferguson BJ, Barnes L, Bernstein JM, Brown D, Clark CE 3rd et al (2000) Geographic variation in allergic fungal rhinosinusitis. Otolaryngol Clin North Am 33:441–449
Montone KT, Livolsi VA, Feldman MD, Palmer J, Chiu AG et al (2012) Fungal rhinosinusitis: a retrospective microbiologic and pathologic review of 400 patients at a single university medical center. Int J Otolaryngol 2012:684835
Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM et al (2011) Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12:1055–1062
Selman M (2004) Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin Chest Med 25:531–547, vi
Solaymani-Dodaran M, West J, Smith C, Hubbard R (2007) Extrinsic allergic alveolitis: incidence and mortality in the general population. QJM 100:233–237
Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE (2001) Hypersensitivity pneumonitis: current concepts. Eur Respir J 32:81s–92s
Kurup VP, Zacharisen MC, Fink JN (2006) Hypersensitivity pneumonitis. Indian J Chest Dis Allied Sci 48:115–128
Ye Q, Nakamura S, Sarria R, Costabel U, Guzman J (2009) Interleukin 12, interleukin 18, and tumor necrosis factor alpha release by alveolar macrophages: acute and chronic hypersensitivity pneumonitis. Ann Allergy Asthma Immunol 102:149–154
Barrera L, Mendoza F, Zuniga J, Estrada A, Zamora AC et al (2008) Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 177:44–55
Bhan U, Newstead MJ, Zeng X, Podsaid A, Goswami M et al (2013) TLR9-dependent IL-23/IL-17 is required for the generation of Stachybotrys chartarum-induced hypersensitivity pneumonitis. J Immunol 190:349–356
Deschamps F, Foudrinier F, Dherbecourt V, Mas P, Prevost E, Legrele AM, Bellier S, Toubas D (2003) Respiratory diseases in French cork workers. Inhal Toxicol 15:1479–1486
Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A et al (2012) Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 129:280–291, quiz 92–3
Von Essen S, Fryzek J, Nowakowski B, Wampler M (1999) Respiratory symptoms and farming practices in farmers associated with an acute febrile illness after organic dust exposure. Chest 116:1452–1458
Perry LP, Iwata M, Tazelaar HD, Colby TV, Yousem SA (1998) Pulmonary mycotoxicosis: a clinicopathologic study of three cases. Mod Pathol 11:4
Flemming J, Hudson B, Rand TG (2004) Comparison of inflammatory and cytotoxic lung responses in mice after intratracheal exposure to spores of two different Stachybotrys chartarum strains. Toxicol Sci 78:267–275
Von Essen SG, Andersen CI, Smith LM (2005) Organic dust toxic syndrome: a noninfectious febrile illness after exposure to the hog barn environment. J Swine Health Prod 13:273–275
Morath SU, Hung R, Bennett JW (2012) Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev 26:73–83
Andersen B, Frisvad JC, Sondergaard I, Rasmussen IS, Larsen LS (2011) Associations between fungal species and water-damaged building materials. Appl Environ Microbiol 77:4180–4188
Brewer JH, Thrasher JD, Hooper D (2014) Chronic illness associated with mold and mycotoxins: is naso-sinus fungal biofilm the culprit? Toxins (Basel) 6:66–80
Hooper DG, Bolton VE, Guilford FT, Straus DC (2009) Mycotoxin detection in human samples from patients exposed to environmental molds. Int J Mol Sci 10:1465–1475
Sardi Jde C, Pitangui Nde S, Rodriguez-Arellanes G, Taylor ML, Fusco-Almeida AM et al (2014) Highlights in pathogenic fungal biofilms. Rev Iberoam Micol 31:22–29
Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A (2012) A water-damaged home and health of occupants: a case study. J Environ Public Health 2012:312836
Yike I, Rand TG, Dearborn DG (2005) Acute inflammatory responses to Stachybotrys chartarum in the lungs of infant rats: time course and possible mechanisms. Toxicol Sci 84:408–417
Claeson AS, Sandstrom M, Sunesson AL (2007) Volatile organic compounds (VOCs) emitted from materials collected from buildings affected by microorganisms. J Environ Monit 9:240–245
Sahlberg B, Gunnbjornsdottir M, Soon A, Jogi R, Gislason T et al (2013) Airborne molds and bacteria, microbial volatile organic compounds (MVOC), plasticizers and formaldehyde in dwellings in three North European cities in relation to sick building syndrome (SBS). Sci Total Environ 444:433–440
Walinder R, Ernstgard L, Norback D, Wieslander G, Johanson G (2008) Acute effects of 1-octen-3-ol, a microbial volatile organic compound (MVOC)—an experimental study. Toxicol Lett 181:141–147
WHO (2009) Guidelines for indoor air quality: dampness and mould. World Heath Organisation, Copenhagen
Smeekens SP, van de Veerdonk FL, Kullberg BJ, Netea MG (2013) Genetic susceptibility to Candida infections. EMBO Mol Med 5:805–813
Wójtowicz A, Bochud PY (2014) Host genetics of invasive Aspergillus and Candida infections. Semin Immunopathol. doi:10.1007/s00281-014-0468-y
Mantovani A, Valentino S, Gentile S, Inforzato A, Bottazzi B et al (2013) The long pentraxin PTX3: a paradigm for humoral pattern recognition molecules. Ann N Y Acad Sci 1285:1–14
Agarwal R, Khan A, Aggarwal AN, Gupta D (2012) Link between CFTR mutations and ABPA: a systematic review and meta-analysis. Mycoses 55:357–365
Vaid M, Kaur S, Sambatakou H, Madan T, Denning DW et al (2007) Distinct alleles of mannose-binding lectin (MBL) and surfactant proteins A (SP-A) in patients with chronic cavitary pulmonary aspergillosis and allergic bronchopulmonary aspergillosis. Clin Chem Lab Med 45:183–186
Brouard J, Knauer N, Boelle PY, Corvol H, Henrion-Caude A et al (2005) Influence of interleukin-10 on Aspergillus fumigatus infection in patients with cystic fibrosis. J Infect Dis 191:1988–1991
Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW et al (2008) Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis 197:618–21
The Nordic Expert Group for Criteria Documentation of Health Risk from Chemicals. Eduard W (2006) Fungal spores. The National Institute for Working Life, Stockholm
Kokkonen M, Ojala L, Parikka P, Jestoi M (2010) Mycotoxin production of selected Fusarium species at different culture conditions. Int J Food Microbiol 143:17–25
Hueza IM, Raspantini PC, Raspantini LE, Latorre AO, Gorniak SL (2014) Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins (Basel) 6:1080–1095
Berek L, Petri IB, Mesterhazy A, Teren J, Molnar J (2001) Effects of mycotoxins on human immune functions in vitro. Toxicol In Vitro 15:25–30
Bracarense AP, Lucioli J, Grenier B, Drociunas Pacheco G, Moll WD et al (2012) Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br J Nutr 107:1776–1786
Ghosh S, Hoselton SA, Dorsam GP, Schuh JM (2013) Eosinophils in fungus-associated allergic pulmonary disease. Front Pharmacol 4:8
Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F (2011) Th17 cells: new players in asthma pathogenesis. Allergy 66:989–998
Knutsen AP, Vijay HM, Kariuki B, Santiago LA, Graff R, Wofford JD et al (2010) Association of IL-4RA single nucleotide polymorphisms, HLA-DR and HLA-DQ in children with Alternaria-sensitive moderate-severe asthma. Clin Mol All 8:5
Porter PC, Lim DJ, Maskatia ZK, Mak G, Tsai CL et al (2014) Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol 134:325–331, e9
Schubert MS, Hutcheson PS, Graff RJ, Santiago L, Slavin RG (2004) HLA-DQB1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J Allergy Clin Immunol 114:1376–1383
Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F et al (2009) Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 182:657–665
Millon L, Roussel S, Rognon B, Quadroni M, Salamin K et al (2012) Aspergillus species recombinant antigens for serodiagnosis of farmer’s lung disease. J Allergy Clin Immunol 130:803–805, e6
Reboux G, Reiman M, Roussel S, Taattola K, Millon L et al (2006) Impact of agricultural practices on microbiology of hay, silage and flour on Finnish and French farms. Ann Agric Environ Med 13:267–273
Jeffery PK (2001) Lymphocytes, chronic bronchitis and chronic obstructive pulmonary disease. Novartis Found Symp 234:149–161, discussion 61–8
Rivoire B, Attucci S, Anthonioz P, Carre P, Lemarie E et al (2001) Occupational acute lung injury due to Alternaria alternata: early stage of organic dust toxic syndrome requires no corticosteroids. Intensive Care Med 27:1236–1237
Acknowledgments
This work has been financially supported by an interdisciplinary grant from the Faculty of Biology and Medicine of the University of Lausanne (Switzerland) to H.N.H. and T.R., the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (France, grant 2011/1/087) to H.N.H., and by the European Community’s Seventh Framework Program [FP7-2007-2013] under grant agreement n° HEALTH-F2-2010-260338–ALLFUN and by a grant from the Swiss National Science Foundation to T.R. (grant 320030_149511).
Conflict of interest
All the authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hélène Niculita-Hirzel and Thierry Roger are joint senior authors
Rights and permissions
About this article
Cite this article
Vacher, G., Niculita-Hirzel, H. & Roger, T. Immune responses to airborne fungi and non-invasive airway diseases. Semin Immunopathol 37, 83–96 (2015). https://doi.org/10.1007/s00281-014-0471-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-014-0471-3