Abstract
Purpose
To examine the pharmacokinetic (PK) profile of several candidate extended-release (ER) formulations of capecitabine in patients.
Methods
In a phase 0 clinical study, PK profiles of several oral candidate ER formulations of capecitabine were compared to the PK profile of capecitabine after administration of the commercially available immediate-release (IR) tablet. A single dose of 1000 mg IR formulation (two 500 mg tablets) was administered on day 1, and a single dose of a 1000 mg candidate ER formulation of capecitabine (two 500 mg tablets) was administered on day 2. Candidate ER formulations of capecitabine differed with regard to the amount of the ER excipient (Kollidon® SR) in tablet matrix (0–5 % w/w) and coating (0–12 mg/cm2).
Results
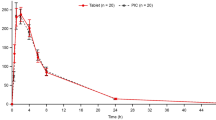
PK profiles of nine different candidate ER formulations were examined. The tablet coating seemed the main determinant for ER of capecitabine and tablet integrity. Average (±standard deviation) AUC0–2h, relative to AUC0–2h after oral administration of the IR tablet, were 43.3 % (±34.9 %) and 1.2 % (±1.2 %) for candidate ER formulations coated with 3 and 6 mg/cm2, respectively. Corresponding AUC0–last were 93.6 % (±40.2 %) and 44.0 % (±5.4 %).
Conclusion
Modulation of capecitabine release in patients can be accomplished by varying tablet coating content. Proof of principle was demonstrated for candidate ER formulations with coating content of 3 mg/cm2.


Similar content being viewed by others
References
Schellens JHM (2007) Capecitabine. Oncologist 12:152–155. doi:10.1634/theoncologist.12-2-152
Midgley R, Kerr DJ (2009) Capecitabine: have we got the dose right? Nat Clin Pract Oncol 6:17–24. doi:10.1038/ncponc1240
Budman DR, Meropol NJ, Reigner B et al (1998) Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 16:1795–1802
Mackean M, Planting A, Twelves C et al (1998) Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 16:2977–2985
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40:85–104
de Bono JS, Twelves CJ (2001) The oral fluorinated pyrimidines. Investig New Drugs 19:41–59
Wilson PM, Danenberg PV, Johnston PG et al (2014) Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol 11:282–298. doi:10.1038/nrclinonc.2014.51
Twelves C, Wong A, Nowacki MP et al (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352:2696–2704. doi:10.1056/NEJMoa043116
Scheithauer W, McKendrick J, Begbie S et al (2003) Oral capecitabine as an alternative to iv 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol 14:1735–1743. doi:10.1093/annonc/mdg500
Mikhail SE, Sun JF, Marshall JL (2010) Safety of capecitabine: a review. Expert Opin Drug Saf 9:831–841. doi:10.1517/14740338.2010.511610
Meta-analysis Group In Cancer (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301–308
Meulenaar J, Beijnen JH, Schellens JHM, Nuijen B (2013) Slow dissolution behaviour of amorphous capecitabine. Int J Pharm 441:213–217. doi:10.1016/j.ijpharm.2012.11.041
Meulenaar J, Keizer RJ, Beijnen JH et al (2013) Development of an extended-release formulation of capecitabine making use of in vitro-in vivo correlation modelling. J Pharm Sci. doi:10.1002/jps.23779
Marchetti S, Schellens JHM (2007) The impact of FDA and EMEA guidelines on drug development in relation to phase 0 trials. Br J Cancer 97:577–581. doi:10.1038/sj.bjc.6603925
Deenen MJ, Rosing H, Hillebrand MJ et al (2013) Quantitative determination of capecitabine and its six metabolites in human plasma using liquid chromatography coupled to electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 913–914:30–40. doi:10.1016/j.jchromb.2012.11.033
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Agnihotri SA, Aminabhavi TM (2006) Novel interpenetrating network chitosan-poly(ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int J Pharm 324:103–115. doi:10.1016/j.ijpharm.2006.05.061
Singh Y, Singh M, Meher JG et al (2014) Trichotomous gastric retention of amorphous capecitabine: an attempt to overcome pharmacokinetic gap. Int J Pharm 478:811–821. doi:10.1016/j.ijpharm.2014.11.055
Acknowledgments
We would like to thank all patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jacobs, B.A., Meulenaar, J., Rosing, H. et al. A phase 0 clinical trial of novel candidate extended-release formulations of capecitabine. Cancer Chemother Pharmacol 77, 1201–1207 (2016). https://doi.org/10.1007/s00280-016-3035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3035-5