Abstract
Purpose
To evaluate the activity and safety of the docetaxel, gemcitabine and bevacizumab combination, administered biweekly, in pretreated patients with HER-2-negative metastatic breast cancer (MBC).
Patients and methods
Women with HER-2-negative MBC, and disease progression after at least one prior line of chemotherapy, were treated with docetaxel 50 mg/m2, gemcitabine 1,500 mg/m2 and bevacizumab 10 mg/kg every 2 weeks. Bevacizumab was continued until disease progression.
Results
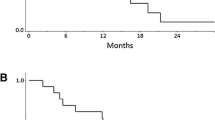
Forty-eight patients have been enrolled. Their median age was 61 years, 95.8 % had a performance status 0–1, 83.3 % had hormone receptor-positive disease, and 47.9 % had received one prior line of chemotherapy. All patients were evaluable for toxicity and 45 for response. Partial response was achieved in 20 patients [PR = 44.4 %, 95 % confidence interval (CI) 29.9–59 %] and disease stabilization in 15 (33.3 %). The median progression-free survival was 7.1 months (95 % CI 4.7–9.5 months) and the median overall survival 21.1 months (95 % CI 10.3–31.9 months). Grade 3–4 neutropenia occurred in 19 patients (39.6 %) and febrile neutropenia in 2 (4.2 %). Most common grade 2–3 non-hematologic adverse events included nausea (10.4 %), diarrhea (10.5 %), neurotoxicity (12.5 %) and fatigue (31.3 %), whereas grade 2 hemorrhage and hypertension occurred in 6.3 and 10.4 %, respectively. There were no grade 4 non-hematologic toxicities or toxic deaths.
Conclusion
The combination of docetaxel, gemcitabine and bevacizumab has promising activity and manageable toxicity as salvage chemotherapy for HER-2-negative MBC patients.

Similar content being viewed by others
References
Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P (2005) Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 104(8):1742–1750. doi:10.1002/cncr.21359
Vogel CL, Azevedo S, Hilsenbeck S, East DR, Ayub J (1992) Survival after first recurrence of breast cancer. The Miami experience. Cancer 70(1):129–135
Hayes DF, Henderson IC, Shapiro CL (1995) Treatment of metastatic breast cancer: present and future prospects. Semin Oncol 22 (2 Suppl 5):5–19; discussion 19–21
Vogel CL (1996) Current status of salvage chemotherapy for refractory advanced breast cancer. Oncology (Williston Park) 10(Suppl 6):7–15
Bissery MC, Guenard D, Gueritte-Voegelein F, Lavelle F (1991) Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res 51(18):4845–4852
Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W (1991) Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res 51(22):6110–6117
Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Schneeweiss A, Lluch A, Llombart A, du Bois A, Kreienberg R, Mayordomo JI, Anton A, Harrison M, Jones A, Carrasco E, Vaury AT, Frimodt-Moller B, Fumoleau P (2009) Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol 27(11):1753–1760. doi:10.1200/JCO.2007.15.8485
Mavroudis D, Malamos N, Alexopoulos A, Kourousis C, Agelaki S, Sarra E, Potamianou A, Kosmas C, Rigatos G, Giannakakis T, Kalbakis K, Apostolaki F, Vlachonicolis J, Kakolyris S, Samonis G, Georgoulias V (1999) Salvage chemotherapy in anthracycline-pretreated metastatic breast cancer patients with docetaxel and gemcitabine: a multicenter phase II trial. Greek Breast Cancer Cooperative Group. Ann Oncol 10(2):211–215
Mavroudis D, Malamos N, Polyzos A, Kouroussis C, Christophilakis C, Varthalitis I, Androulakis N, Kalbakis K, Milaki G, Georgoulias V (2004) Front-line chemotherapy with docetaxel and gemcitabine administered every two weeks in patients with metastatic breast cancer: a multicenter phase II study. Oncology 67(3–4):250–256. doi:10.1159/000081325
Karachaliou N, Kouroussis C, Papakotoulas P, Kalbakis K, Tryfonidis K, Vardakis N, Poppis E, Georgoulias V, Mavroudis D (2012) A multicenter phase II trial of docetaxel plus gemcitabine as salvage treatment in anthracycline- and taxane-pretreated patients with metastatic breast cancer. Cancer Chemother Pharmacol 69(5):1345–1352. doi:10.1007/s00280-012-1824-z
Alexopoulos A, Tryfonopoulos D, Karamouzis MV, Gerasimidis G, Karydas I, Kandilis K, Stavrakakis J, Stavrinides H, Georganta C, Ardavanis A, Rigatos G (2004) Evidence for in vivo synergism between docetaxel and gemcitabine in patients with metastatic breast cancer. Ann Oncol 15(1):95–99
Schneider BP, Miller KD (2005) Angiogenesis of breast cancer. J Clin Oncol 23(8):1782–1790. doi:10.1200/JCO.2005.12.017
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. doi:10.1038/nm0603-669
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676. doi:10.1056/NEJMoa072113
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23(4):792–799. doi:10.1200/JCO.2005.05.098
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29(32):4286–4293. doi:10.1200/JCO.2010.34.1255
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Cancer Therapy Evaluation Program (2006) Common toxicity criteria. Version 3.0. DCTD, NCI, NIH, DHHS
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Collet D (1994) Modelling survival data in medical research, 3rd edn. Blackwell Scientific, Oxford
Cox DR (1970) The analysis of binary data, 1st edn. Methuen, London
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28(20):3239–3247. doi:10.1200/JCO.2008.21.6457
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29(10):1252–1260. doi:10.1200/JCO.2010.28.0982
Fountzilas G, Nicolaides C, Bafaloukos D, Kalogera-Fountzila A, Kalofonos H, Samelis G, Aravantinos G, Pavlidis N (2000) Docetaxel and gemcitabine in anthracycline-resistant advanced breast cancer: a Hellenic Cooperative Oncology Group Phase II study. Cancer Invest 18(6):503–509
Kornek GV, Haider K, Kwasny W, Raderer M, Schull B, Payrits T, Depisch D, Kovats E, Lang F, Scheithauer W (2002) Treatment of advanced breast cancer with docetaxel and gemcitabine with and without human granulocyte colony-stimulating factor. Clin Cancer Res 8(5):1051–1056
Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL (2009) Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol 27(30):4966–4972. doi:10.1200/JCO.2008.21.6630
Brufsky A, Hoelzer K, Beck T, Whorf R, Keaton M, Nadella P, Krill-Jackson E, Kroener J, Middleman E, Frontiera M, Paul D, Panella T, Bromund J, Zhao L, Orlando M, Tai F, Marciniak MD, Obasaju C, Hainsworth J (2011) A randomized phase II study of paclitaxel and bevacizumab with and without gemcitabine as first-line treatment for metastatic breast cancer. Clin Breast Cancer 11(4):211–220. doi:10.1016/j.clbc.2011.03.019
Khoo KS, Manzoor Zaidi SH, Srimuninnimit V, Song S, Nair R, Ngelangel CA, Bustam A, Reece WH, Lehnert M (2006) Gemcitabine and split-dose paclitaxel or docetaxel in metastatic breast cancer: a randomised phase II study. Eur J Cancer 42(12):1797–1806. doi:10.1016/j.ejca.2006.05.001
Del Mastro L, Fabi A, Mansutti M, De Laurentiis M, Durando A, Merlo DF, Bruzzi P, La Torre I, Ceccarelli M, Kazeem G, Marchi P, Boy D, Venturini M, De Placido S, Cognetti F (2013) Randomised phase 3 open-label trial of first-line treatment with gemcitabine in association with docetaxel or paclitaxel in women with metastatic breast cancer: a comparison of different schedules and treatments. BMC Cancer 13:164. doi:10.1186/1471-2407-13-164
Colozza M, de Azambuja E, Personeni N, Lebrun F, Piccart MJ, Cardoso F (2007) Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist 12(3):253–270. doi:10.1634/theoncologist.12-3-253
Kosmas C, Tsavaris N, Mylonakis N, Kalofonos HP (2003) An overview of current results with the gemcitabine and docetaxel combination as initial and salvage chemotherapy regimen in advanced non-small cell lung cancer. Crit Rev Oncol Hematol 45(3):265–275
Montero AJ, Avancha K, Gluck S, Lopes G (2012) A cost-benefit analysis of bevacizumab in combination with paclitaxel in the first-line treatment of patients with metastatic breast cancer. Breast Cancer Res Treat 132(2):747–751. doi:10.1007/s10549-011-1919-y
Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V (2013) AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol 31(14):1719–1725. doi:10.1200/JCO.2012.44.7912
Jubb AM, Harris AL (2010) Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11(12):1172–1183. doi:10.1016/S1470-2045(10)70232-1
Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD (2008) Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26(28):4672–4678. doi:10.1200/JCO.2008.16.1612
Acknowledgments
This work was partially supported by a research Grant from the Cretan Association for Biomedical Research (CABR). Dr E. K. was a recipient of a CABR clinical fellowship.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kontopodis, E., Kentepozidis, N., Christophyllakis, C. et al. Docetaxel, gemcitabine and bevacizumab as salvage chemotherapy for HER-2-negative metastatic breast cancer. Cancer Chemother Pharmacol 75, 153–160 (2015). https://doi.org/10.1007/s00280-014-2628-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2628-0