Abstract
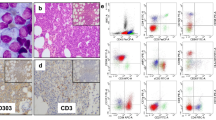
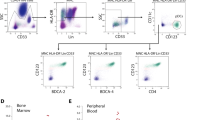
Two distinct forms of neoplasms derived from plasmacytoid dendritic cells (PDC) exist: mature PDC proliferations associated with myeloid neoplasms and blastic PDC neoplasms (BPDCN). Ten cases of PDC proliferations and neoplasms in the bone marrow have been submitted to the bone marrow workshop held at the 18th EAHP meeting. Based on observations from the submitted cases, scattered PDC (≤1% of cells) and PDC aggregates (≤10 PDC/HPF) reflect the normal bone marrow composition, while in myelodysplastic syndromes (MDS), there is a propensity for larger/more PDC aggregates (1–5% and 35 PDC/HPF). A shared PTPN11 mutation between a mature PDC proliferation and an accompanying MDS provides evidence of clonal relationship in such instances and shows that PDC are a part of the malignant clone. CD123 and CD303 should be considered backbone markers to histopathologically establish the diagnosis of BPDCN, since they are detectable in almost all cases and properly well on biopsies subjected to different fixations. Expression of some T-cell markers (e.g., CD2 and CD7 but not CD3), B-cell markers (e.g., CD79a but not CD19 and CD20), and myeloid markers (e.g., CD33 and CD117 but not myeloperoxidase) can be observed in BPDCN. Genetical data of the summarized cases corroborate the important role of chromosomal losses in BPDCN. Together with five previously reported instances, one additional workshop case with MYC rearrangement proposes that translocations of MYC may be recurrent. The frequent nature of deleterious mutations of IKZF3 and deletions of IKZF1 suggests a role for the Ikaros family proteins in BPDCN.







Similar content being viewed by others
References
Lennert K, Remmele W (1958) Karyometrische Untersuchungen an Lymphknotenzellen des Menschen: I. Mitt. Germinoblasten, Lymphoblasten und Lymphozyten. Acta Haematol (Basel) 19:99–113
Grouard G, Rissoan MC, Filgueira L et al (1997) The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 185:1101–1111
Horny HP, Feller AC, Horst HA, Lennert K (1987) Immunocytology of plasmacytoid T cells: marker analysis indicates a unique phenotype of this enigmatic cell. Hum Pathol 18:28–32
Cao W (2009) Molecular characterization of human plasmacytoid dendritic cells. J Clin Immunol 29:257–264
Cella M, Jarrossay D, Facchetti F et al (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 5:919–923
Mathan TS, Figdor CG, Buschow SI (2013) Human plasmacytoid dendritic cells: from molecules to intercellular communication network. Front Immunol 4:372
Decalf J, Fernandes S, Longman R et al (2007) Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med 204:2423–2437
Liu YJ (2005) IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23:275–306
Penna G, Vulcano M, Roncari A et al (2002) Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol 169:6673–6676
Sozzani S, Vermi W, Del Prete A, Facchetti F (2010) Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol 31:270–277
Vermi W, Lonardi S, Morassi M et al (2009) Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology 214:877–886
Vermi W, Soncini M, Melocchi L, Sozzani S, Facchetti F (2011) Plasmacytoid dendritic cells and cancer. J Leukoc Biol 90:681–690
Colonna M, Trinchieri G, Liu YJ (2004) Plasmacytoid dendritic cells in immunity. Nat Immunol 5:1219–1226
Charles J, Chaperot L, Salameire D et al (2010) Plasmacytoid dendritic cells and dermatological disorders: focus on their role in autoimmunity and cancer. Eur J Dermatol 20:16–23
Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V (2011) Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 29:163–183
Swiecki M, Colonna M (2015) The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15:471–485
Liu K, Victora GD, Schwickert TA et al (2009) In vivo analysis of dendritic cell development and homeostasis. Science 324:392–397
Naik SH, Sathe P, Park HY et al (2007) Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8:1217–1226
Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG (2003) Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med 198:305–313
Maraskovsky E, Daro E, Roux E et al (2000) In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96:878–884
Cisse B, Caton ML, Lehner M et al (2008) Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135:37–48
Boiocchi L, Lonardi S, Vermi W, Fisogni S, Facchetti F (2013) BDCA-2 (CD303): a highly specific marker for normal and neoplastic plasmacytoid dendritic cells. Blood 122:296–297
Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D (2003) TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood 101:5007–5009
Facchetti F, Cigognetti M, Fisogni S et al (2016) Neoplasms derived from plasmacytoid dendritic cells. Mod Pathol 29:98–111
Facchetti F, de Wolf-Peeters C, Mason DY et al (1988) Plasmacytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin. Am J Pathol 133:15–21
Facchetti F, Vermi W, Mason D, Colonna M (2003) The plasmacytoid monocyte/interferon producing cells. Virchows Arch 443:703–717
Dargent JL, Delannoy A, Pieron P et al (2011) Cutaneous accumulation of plasmacytoid dendritic cells associated with acute myeloid leukemia: a rare condition distinct from blastic plasmacytoid dendritic cell neoplasm. J Cutan Pathol 38:893–898
Marafioti T, Paterson JC, Ballabio E et al (2008) Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood 111:3778–3792
Facchetti F, De Wolf-Peeters C, van den Oord JJ, De vos R, Desmet VJ (1988) Plasmacytoid T cells: a cell population normally present in the reactive lymph node. An immunohistochemical and electronmicroscopic study. Hum Pathol 19:1085–1092
Vermi W, Facchetti F, Rosati S et al (2004) Nodal and extranodal tumor-forming accumulation of plasmacytoid monocytes/interferon-producing cells associated with myeloid disorders. Am J Surg Pathol 28:585–595
Vitte F, Fabiani B, Benet C et al (2012) Specific skin lesions in chronic myelomonocytic leukemia: a spectrum of myelomonocytic and dendritic cell proliferations: a study of 42 cases. Am J Surg Pathol 36:1302–1316
Benet C, Gomez A, Aguilar C et al (2011) Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol 135:278–290
Horny HP, Kaiserling E, Handgretinger R et al (1995) Evidence for a lymphotropic nature of circulating plasmacytoid monocytes: findings from a case of CD56+ chronic myelomonocytic leukemia. Eur J Haematol 54:209–216
Facchetti F, Jones DM, Petrella T (2008) Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. International Agency for Research on Cancer, Lyon, France, pp 145–147
Chopin M, Preston SP, Lun AT et al (2016) RUNX2 mediates plasmacytoid dendritic cell egress from the bone marrow and controls viral immunity. Cell Rep 15:866–878
Hirohata S, Yanagida T, Tomita T, Yoshikawa H (2014) Increased generation of pre-plasmacytoid dendritic cells in bone marrow of rheumatoid arthritis. Mod Rheumatol 24:443–447
Jegalian AG, Facchetti F, Jaffe ES (2009) Plasmacytoid dendritic cells. Physiologic roles and pathologic states. Adv Anat Pathol 16:392–404
Fitzgerald-Bocarsly P, Dai J, Singh S (2008) Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 19:3–19
Ito T, Wang YH, Liu YJ (2005) Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol 26:221–229
Kadowaki N, Ho S, Antonenko S et al (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194:863–870
von Landenberg P, Bauer S (2007) Nucleic acid recognizing toll-like receptors and autoimmunity. Curr Opin Immunol 19:606–610
Vallin H, Blomberg S, Alm GV, Cederblad B, Rönnblom L (1999) Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leukocytes resembling immature dendritic cells. Clin Exp Immunol 115:196–202
Banchereau J, Pascual V (2006) Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25:383–392
Cederblad B, Blomberg S, Vallin H et al (1998) Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha producing cells. J Autoimmun 11:465–470
Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL (2001) Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 159:237–243
Wollenberg A, Wagner M, Gunther S et al (2002) Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol 119:1096–1102
Santoro A, Majorana A, Roversi L et al (2005) Recruitment of dendritic cells in oral lichen planus. J Pathol 205:426–434
Facchetti F, de Wolf-Peeters C, van den Oord JJ et al (1989) Plasmacytoid monocytes (so-called plasmacytoid T-cells) in Kikuchi's lymphadenitis. An immunohistologic study. Am J Clin Pathol 92:42–50
Harris NL, Bhan AK (1987) “Plasmacytoid T cells” in Castleman's disease. Immunohistologic phenotype. Am J Surg Pathol 11:109–113
Munn DH, Sharma MD, Hou D et al (2004) Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 114:280–290
Sharma MD, Baban B, Chandler P et al (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 117:2570–2582
Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR (2008) The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 181:5396–5404
Weissmann S, Alpermann T, Grossmann V et al (2012) Landscape of TET2 mutations in acute myeloid leukemia. Leukemia 26:934–942
Orazi A, Chiu R, O'Malley DP et al (2006) Chronic myelomonocytic leukemia: the role of bone marrow biopsy immunohistology. Mod Pathol 19:1536–1545
Harris NL, Demirjian Z (1991) Plasmacytoid T-zone cell proliferation in a patient with chronic myelomonocytic leukemia. Histologic and immunohistologic characterization. Am J Surg Pathol 15:87–95
Chen YC, Chou JM, Ketterling RP, Letendre L, Li CY (2003) Histologic and immunohistochemical study of bone marrow monocytic nodules in 21 cases with myelodysplasia. Am J Clin Pathol 120:874–881
Pileri SA, Ascani S, Cox MC et al (2007) Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 21:340–350
Bodmer A, Menter T, Juskevicius D et al (2017) Sharing of a PTPN11 mutation by myelodysplastic bone marrow and a mature plasmacytoid dendritic cell proliferation provides evidence for their common myelomonocytic origin. Virchows Arch. doi:10.1007/s00428-017-2075-5
Puda A, Milosevic JD, Berg T et al (2012) Frequent deletions of JARID2 in leukemic transformation of chronic myeloid malignancies. Am J Hematol 87:245–250
Tartaglia M, Niemeyer CM, Fragale A et al (2003) Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet 34:148–150
Bentires-Alj M, Paez JG, David FS et al (2004) Activating mutations of the Noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res 64:8816–8820
Hugues L, Cavé H, Philippe N et al (2005) Mutations of PTPN11 are rare in adult myeloid malignancies. Haematologica 90:853–854
Takiuchi Y, Maruoka H, Aoki K et al (2012) Leukemic manifestation of blastic plasmacytoid dendritic cell neoplasm lacking skin lesion: a borderline case between acute monocytic leukemia. J Clin Exp Hematop 52:107–111
Tallman MS, Hakimian D, Shaw JM et al (1993) Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia. J Clin Oncol 11:690
Novotny JR, Nückel H, Dührsen U (2006) Correlation between expression of CD56/NCAM and severe leukostasis in hyperleukocytic acute myelomonocytic leukaemia. Eur J Haematol 76:299–308
Pagano L, Valentini CG, Pulsoni A et al (2013) Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 98:239–246
Pagano L, Valentini CG, Grammatico S, Pulsoni A (2016) Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol 174:188–202
Torlakovic EE, Brynes RK, Hyjek E et al (2015) ICSH guidelines for the standardization of bone marrow immunohistochemistry. Int J Lab Hematol 37:431–449
Nakamura Y, Kayano H, Kakegawa E et al (2015) Identification of SUPT3H as a novel 8q24/MYC partner in blastic plasmacytoid dendritic cell neoplasm with t(6;8)(p21;q24) translocation. Blood Cancer Journal 5:e301
Sapienza MR, Fuligni F, Agostinelli C et al (2014) Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 28:1606–1616
Arranto CA, Tzankov A, Halter J (2017) Blastic plasmacytoid dendritic cell neoplasm with transient response to pralatrexate. Ann Hematol. doi:10.1007/s00277-016-2907-4
Montero J, Stephansky J, Cai T et al (2016) Blastic plasmacytoid dendritic cell neoplasm is dependent on BCL2 and sensitive to venetoclax. Cancer Discov. doi:10.1158/2159-8290.CD-16-0999
Leroux D, Mugneret F, Callanan M et al (2002) CD4(+), CD56 (+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Francais de Cytogenetique Hematologique. Blood 99:4154–4159
Lucioni M, Novara F, Fiandrino G et al (2011) Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood 118:4591–4594
Wiesner T, Obenauf AC, Cota C et al (2010) Alterations of the cell-cycle inhibitors p27(KIP1) and p16(INK4a) are frequent in blastic plasmacytoid dendritic cell neoplasms. J Invest Dermatol 130:1152–1157
Cao Q, Liu F, Niu G, Xue L, Han A (2014) Blastic plasmacytoid dendritic cell neoplasm with EWSR1 gene rearrangement. J Clin Pathol 67:90–92
Gao NA, Wang XX, Sun JR, Yu WZ, Guo NJ (2015) Blastic plasmacytoid dendritic cell neoplasm with leukemic manifestation and ETV6 gene rearrangement: a case report. Exp Ther Med 9:1109–1112
Toya T, Nishimoto N, Koya J et al (2012) The first case of blastic plasmacytoid dendritic cell neoplasm with MLL-ENL rearrangement. Leuk Res 36:117–118
Tokuda K, Eguchi-Ishimae M, Yagi C et al (2014) CLTC-ALK fusion as a primary event in congenital blastic plasmacytoid dendritic cell neoplasm. Genes Chromosomes Cancer 53:78–89
Yang N, Huh J, Chung WS et al (2015) KMT2A (MLL)-MLLT1 rearrangement in blastic plasmacytoid dendritic cell neoplasm. Cancer Genet 208:464–467
Cook JR, Shekhter-Levin S, Swerdlow SH (2004) Utility of routine classical cytogenetic studies in the evaluation of suspected lymphomas: results of 279 consecutive lymph node/extranodal tissue biopsies. Am J Clin Pathol 121:826–835
Sreekantaiah C, Leong SPL, Karakousis CP et al (1991) Cytogenetic profiles of 109 lipomas. Cancer Res 51:422–433
Panagopoulos I, Mertens F, Isaksson M et al (2002) Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer 35:340–352
Dietrich CU, Krone W, Hochsattel R (1990) Cytogenetic studies in tuberous sclerosis. Cancer Genet Cytogenet 45:161–177
Aspberg F, Mertens F, Bauer HC et al (1995) Near-haploidy in two malignant fibrous histiocytomas. Cancer Genet Cytogenet 79:119–122
Fletcher CDM, Akerman M, Dal Cin P et al (1996) Correlation between clinicopathological features and karyotype in lipomatous tumors: a report of 178 cases from the chromosomes and morphology (CHAMP) collaborative study group. Am J Pathol 148:623–630
Gisselsson D, Hibbard MK, Dal Cin P et al (2011) PLAG1 alterations in lipoblastoma: involvement in varied mesenchymal cell types and evidence for alternative oncogenic mechanisms. Am J Pathol 159:955–962
Bergman D, Halje M, Nordin M, Engström W (2013) Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology 59:240–249
Alayed K, Patel KP, Konoplev S et al (2013) TET2 mutations, myelodysplastic features, and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am J Hematol 88:1055–1061
Jardin F, Ruminy P, Parmentier F et al (2011) TET2 and TP53 mutations are frequently observed in blastic plasmacytoid dendritic cell neoplasm. Br J Haematol 153:413–416
Stenzinger A, Endris V, Pfarr N et al (2014) Targeted ultradeep sequencing reveals recurrent and mutually exclusive mutations of cancer genes in blastic plasmacytoid dendritic cell neoplasm. Oncotarget 5:6404–6413
Menezes J, Acquadro F, Wiseman M et al (2014) Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia 28:823–829
Schulze K, Imbeaud S, Letouzé E et al (2015) Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 47:505–511
Kastner P, Chan S (2011) Role of Ikaros in T-cell acute lymphoblastic leukemia. World J Biol Chem 2:108–114
Mullighan CG, Miller CB, Radtke I (2008) BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453:110–114
Trageser D, Iacobucci I, Nahar R et al (2009) Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J Exp Med 206:1739–1753
Iacobucci I, Storlazzi CT, Cilloni D et al (2009) Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell'Adulto Acute Leukemia Working Party (GIMEMA AL WP). Blood 114:2159–2167
Holmfeldt L, Wei L, Diaz-Flores E et al (2013) The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 45:242–252
Acknowledgements
A.T. thanks Prof. Stephan Dirnhofer from the Institute of Pathology at the University Hospital of Basel for putting his immeasurable efforts to organize and host the 18th EAHP meeting in Basel; Petra Huber from the Institute of Pathology at the University Hospital of Basel for her inestimable logistic sustenance; and Rumyana Todorova, a talented young member of the European Bone Marrow Working Group, for her enthusiastic support at the initial examination of all cases submitted for the bone marrow workshops—such young people invigorate and inspire our working group.
The panel acknowledges the case submitters Dr. Corina Dommann-Scherrer from Winterthur, Switzerland (case 103); Dr. Jie Xu from Houston, USA (case 134); Dr. Juehua Gao from Chicago, USA (case 143); Dr. Lauren Smith from Ann Arbor, USA (case 190); Dr. Vathany Sriganeshan from Miami, USA (case 222); Dr. Raymond E. Felgar✞ from Pittsburgh, USA (case 272); Dr. Prashanti Reddy from Carlsbad, USA (case 296); Dr. Konnie Hebeda from Nijmegen, the Netherlands (case 303); Dr. Alexandar Tzankov from Basel, Switzerland (case 310); and Dr. Rahul Matnani from New York, USA (case 184).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tzankov, A., Hebeda, K., Kremer, M. et al. Plasmacytoid dendritic cell proliferations and neoplasms involving the bone marrow. Ann Hematol 96, 765–777 (2017). https://doi.org/10.1007/s00277-017-2947-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2947-4