Abstract
Introduction
We hypothesize that the combination of transarterial embolization (TAE) plus inhibition of lactate export will limit anaerobic metabolism and reduce tumor survival compared to TAE alone. The purpose of this study was to test this hypothesis in a rat model of hepatocellular carcinoma (HCC).
Methods
Rat N1-S1 hepatoma cells were assayed in vitro using the Seahorse XF analyzer to measure extracellular acidification (lactate excretion) comparing effects of the addition of caffeic acid (CA) or ferulic acid (FA) or UK-5099 with control. Monocarboxylate transporter Slc16a3 was knocked down by RNAi. N1S1 tumors were orthotopically implanted in rats and 4 groups evaluated: (1) Control, (2) TAE-only, (3) TAE plus CA, and (4) TAE plus FA. Tumor size was determined by ultrasound and analyzed by repeated measures statistics. Tumors harvested at 4 weeks were examined by microscopy.
Results
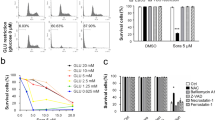
Seahorse assays showed that CA and FA caused a significant reduction by >90% in lactate efflux by N1S1 tumor cells (p < 0.01). Knockdown of Slc16a3 prevented inhibition by CA. In vivo tumors grew 30-fold in volume over 4 weeks in untreated controls. By comparison, TAE resulted in near cessation of growth (10% in 4-week time period). However, both TAE + CA and TAE + FA caused a significant reduction of tumor volumes (87 and 72%, respectively) compared to control and TAE (p < 0.05). Pathologic evaluation revealed residual tumor in the TAE group but no residual viable tumor cells in the TAE + CA and TAE + FA groups.
Conclusion
Addition of CA or FA enhances the effectiveness of TAE therapy for HCC in part by blocking lactate efflux.





Similar content being viewed by others
References
Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004;5:409–18.
Asghar U, Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol. 2012;56:686–95.
Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365–76.
Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Interv Radiol. 2007;30:6–25.
Johnson CG, Sharma KV, Levy EB, Woods DL, Morris AH, Bacher JD, et al. Microvascular perfusion changes following transarterial hepatic tumor embolization. J Vasc Interv Radiol. 2016;27:133–41.
Mathupala SP, Colen CB, Parajuli P, Sloan AE. Lactate and malignant tumors: a therapeutic target at the end stage of glycolysis. J Bioenerg Biomembr. 2007;39(1):73–7.
Kang KW, Jin MJ, Han HK. IGF-I receptor gene activation enhanced the expression of monocarboxylic acid transporter 1 in hepatocarcinoma cells. Biochem Biophys Res Commun. 2006;342(4):1352–5.
Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by a-cyano-4-hydroxycinnamate. Biochem J. 1974;138:313–6.
Halestrap AP, Meredith D. The Slc16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflug Arch. 2004;447:619–28.
Spencer TL, Lehninger AL. L-lactate transport in ehrlich ascites-tumour cells. Biochem J. 1976;154:405–14.
Wu YC, Hseih CL. Pharmacological effects of Radix Angelica Sinensis (Danggui) on cerebral infarction. Chin Med. 2011;6:1–5.
Gamaro GD, Suyenga E, Borsoi M, Lerman J, Pereira P, Ardenghi P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol. 2011. doi:10.5402/2011/451682.
Krix M, Kiessling F, Vosseler S, Kiessline I, Le-Huu M, Fusenig NE, et al. Comparison of intermittent-bolus contrast imaging with conventional power Doppler sonography: quantification of tumor perfusion in small animals. Ultrasound Med Biol. 2003;29:1093–103.
Rubin D. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987.
Patterson JN, Cousteils K, Lou JW, Fox JEM, MacDonald PE, Joseph JW. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. J Biol Chem. 2014;289:13335–46.
Nakasima RA, Paggi MG, Pedersen PL. Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984;44:5702–6.
Van Heiden MG. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Weinberg R. pRb and Control of the Cell Cycle Clock. In: Weinberg R, editor. 2007 Biology of Cancer. New York: Garland Science; 2007. p. 255–306.
Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, et al. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience. 2014;1:777–802.
Leite TC, Coelho RG, Da Silva D, Coelho WS, Marinho-Carvalho MM, Sola-Penna M. Lactate down regulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Lett. 2011;585(1):92–8.
Lao MS, Toth D. Effects of ammonium and lactate on growth and metabolism of a recombinant Chinese hamster ovary cell culture. Biotechnol Prog. 2008;13(5):688–91.
Marx E, Mueller-Klieser W, Vaupel P. Lactate-induced inhibition of tumor cell proliferation. Int J Radiat Oncol Biol Phys. 1988;14(5):947–55.
Cruz HJ, Freitas CM, Alves PM, Moreira JL, Carrondo MJT. Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzyme Microb Technol. 2000;27(1–2):43–52.
Ozturk SS, Riley MR, Palsson BO. Effects of ammonia and lactate on hybridoma growth, metabolism, and antibody production. Biotechnol Bioeng. 1992;39(4):418–31.
Sattler UG, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C, et al. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol. 2010;94(1):102–9.
Quennet V, Yaromina A, Zips D, Rosner A. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol. 2006;81(2):130–5.
Haaga J, Haaga R. Acidic lactate sequentially induced lymphogenesis, phlebogenesis, and arteriogenesis (ALPHA) hypothesis: Lactate-triggered glycolytic vasculogenesis that occurs in normoxia or hypoxia and complements the traditional concept of hypoxia-based vasculogenesis. Surgery. 2013;154:632–7.
Prasad RN, Karthikeyan A, Karthikeyan S, Reddy BV. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcome cell line. Mol Cell Biochem. 2011;349:11–9.
Acknowledgements
The authors wish to thank Mark Conway, PhD for his assistance in the statistical analysis of this study.
Funding
The research presented in this manuscript was supported by the Thelma R. Swortzel Collaborative Research Grant from the University of Virginia, the Paul Mellon Institute of the University of Virginia, and a grant from the NCI, NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed Consent
Informed consent is not required for this study.
Rights and permissions
About this article
Cite this article
Wilkins, L.R., Brautigan, D.L., Wu, H. et al. Cinnamic Acid Derivatives Enhance the Efficacy of Transarterial Embolization in a Rat Model of Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 40, 430–437 (2017). https://doi.org/10.1007/s00270-016-1515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-016-1515-y