Abstract
Background
The prognosis of hepatocellular carcinoma (HCC) with macroscopic vascular invasion is extremely poor even after hepatic resection. We aimed to clarify the efficacy of adjuvant hepatic arterial infusion chemotherapy (HAI) for HCC with vascular invasion.
Methods
A total of 73 HCC patients with macroscopic vascular invasion were divided into two groups: 38 with hepatectomy with HAI (HAI group) and 35 with hepatectomy alone (non-HAI group). From 1997 to 2007, HAI was performed via an implanted injection port. The treatment comprised three courses of weekly infusion of HAI, which comprised cisplatin (10 mg daily on days 1–5) followed by 5-fluorouracil (5-FU; 250 mg daily on days 1–5) infusion. From 2007, cisplatin (60 mg/m2), 5-FU (600 mg/m2), and a mixture of mitomycin C (3 mg/m2) and degradable starch microspheres were administered for two courses.
Results
Overall, 92 % of patients completed adjuvant HAI. In the HAI and non-HAI groups, the 5-year disease-free survival (DFS) rates were 33.1 % and 11.8 %, respectively (p = 0.029), and the 5-year overall survival (OS) rates were 46.7 % and 32.7 %, respectively (p = 0.318). Among the patients with Vp3/4 or Vv3 (n = 32) in the HAI group, the 3-year DFS and OS rates were 33.7 % and 56.8 %, respectively (p = 0.049). Those in the non-HAI group were 8.3 % and 12.0 %, respectively (p = 0.023). Cox proportional multivariate analysis for DFS revealed that HAI was an independent favorable prognostic factor in all 73 patients (hazard ratio 0.536; p = 0.029).
Conclusions
Adjuvant HAI for HCC patients with vascular invasion might reduce the risk of recurrence.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide [1]. Hepatectomy achieves favorable outcomes in well-selected candidates and is still considered one of the most potentially curative treatments for HCC [2]. Unfortunately, the long-term survival after hepatectomy is unsatisfactory because of the high incidence of tumor recurrence, especially intrahepatic tumors [3]. After curative hepatic resection for HCC, the recurrence rates at 2 and 5 years are approximately 50 % to 60 % and 80 %, respectively [4–6]. The prognosis remains extremely poor in those with advanced HCC with distinct vascular invasion, such as tumor thrombosis of the first branch or trunk of the portal vein or inferior vena cava [7, 8]. Portal vein tumor thrombosis (PVTT) can cause widespread dissemination of tumor cells via the portal tract as well as liver dysfunction and portal vein hypertension. In turn, this can lead to intractable ascites, variceal rupture, hepatic encephalopathy, and/or death [9]. The risks of PVTT with intrahepatic dissemination after local ablation therapy—e.g., ethanol injection therapy, microwave coagulation therapy, radiofrequency ablation—for small HCCs adjacent to the main or sectional portal vein were recently reported [10]. However, even with the removal of a macroscopic PVTT, there may still be microscopic tumor thrombi, which can be the source of a recurrence. Thus, an effective adjuvant therapy is required to prevent tumor recurrence after hepatic resection treatments for patients with vascular invasion.
Recent advances in implantable drug delivery systems have facilitated repeated hepatic arterial infusion of chemotherapy (HAI). Several studies report that intraarterial 5-fluorouracil (5-FU) with low-dose cisplatin [7, 11, 12] or 5-FU and systemic interferon (IFNα) [13] are the most effective combinations for unresectable HCCs with PVTT. However, there is little convincing evidence indicating that adjuvant therapy reduces the risk of recurrence after hepatic resection. Furthermore, no standard regimen has been established.
In the present study, we assessed the efficacy and feasibility of intraarterial cisplatin/5-FU combination therapy for surgically resected HCCs with massive vascular invasion. We identified patients subsets who would most likely benefit from adjuvant HAI.
Methods
From April 1997 to March 2011, a total of 539 patients underwent hepatic resection for HCC at Kumamoto University Hospital. Among them, the 77 patients who underwent resection of HCC with macroscopic vascular invasion (≥Vp2 or ≥Vv2) and without distant metastasis were enrolled in this study. The invasion sites of portal venous invasion (Vp) and hepatic venous invasion (Vv) were defined as follows: Vp2, second-order branches of the portal vein; Vp3, first-order branches of the portal vein; Vp4, main trunk or opposite the first branch of the portal vein; Vv2, main trunk of the hepatic vein; Vv3, inferior vena cava. Portal or hepatic venous invasion was diagnosed based on the findings of preoperative imaging studies, such as dynamic computed tomography (CT) or magnetic resonance imaging (MRI) and CT angiography. The diagnosis of HCC was confirmed by histopathological examinations of the resected specimens.
The entry criteria of HAI group were as follows: no recurrence confirmed 1 month after hepatic resection; appropriate liver function reserve; good performance status; sufficient recovery from the operation. Four patients died because of postoperative complications (liver failure 2; acute respiratory distress syndrome 1; intraabdominal infection 1) and were excluded from analysis. The remaining 73 patients were reviewed retrospectively. In all, 38 patients treated with hepatic resection and HAI were allocated to the HAI group. During the same period, 35 patients who underwent hepatic resection without HAI therapy by the same surgical team were allocated to the non-HAI group. The reasons why patients did not receive HAI were as follows: early recurrence (n = 10), disapproval of the therapy (n = 10), advanced age (n = 4), co-morbidities (n = 7), poor performance status (n = 4).
Patients diagnosed with multiple and hypervascular tumor recurrence in the remnant liver on CT angiography 1 month after hepatic resection (n = 10) were treated with transarterial chemoembolization (TACE) using cisplatin suspended in Lipiodol with a gelatin sponge instead of HAI according to our treatment strategy [14]. This study was conducted in accordance with the Declaration of Helsinki and the ethical guidelines for clinical studies of the Ministry of Health, Labor, and Welfare in Japan. Written informed consent was obtained from all patients.
Surgical technique
All patients underwent hepatic resection performed by two senior liver surgeons. The operative procedure was determined beforehand on the basis of the liver function reserve, the extent of the main and satellite tumors, and portal or venous invasion. Parenchymal dissection was performed using an ultrasonic surgical aspirator (CUSA; Valley Lab, Boulder, CO, USA) and bipolar forceps with intermittent clamping of the portal triad. After 2005, we preferred a precoagulation technique using a dissecting sealer (Valley Lab) or the VIO soft coagulation system (ERBE, Elektromedizin GmbH, Tübingen, Germany) [15]. For the patients with Vp4, the PVTT was removed using the “peeling off” technique [16]. In brief, the portal venotomy was placed after adequate vascular control of the portal flow was established. The PVTT was dissected from the portal venous wall and removed via the opening. Macroscopic residual PVTTs intruding into tiny branches were extracted meticulously. A multiperforated drain was placed in the abdominal cavity at the end of the procedure.
HAI treatment
From 1997 to 2007, patients with HCCs were treated by arterial infusion of a chemotherapeutic agent via a subcutaneously implanted injection port (old protocol). In principle, a hepatic arterial catheter was placed 2 to 3 weeks after the operation via the femoral artery. Celiac angiography was performed according to the Seldinger technique. A 4For 5F heparin-coated catheter was introduced into the proper or common hepatic artery. The gastroduodenal and right gastric arteries were occluded using a steel coil to prevent gastroduodenal injury caused by anticancer agents. After the catheter was connected to the injection port, the device was implanted in a subcutaneous pocket in the right lower quadrant to avoid catheter kinks. One course consisted of cisplatin administration (10 mg daily on days 1–5) and subsequent infusion of 5-FU (250 mg daily on days 1–5). After confirming a lack of recurrence in the remnant liver by CT angiography 4 weeks after surgery, HAI was started and repeated for 3 courses. The injection port was removed 1 week after the end of HAI with confirmation of a lack of recurrence.
From 2007, patients with HCC were treated without an injection port (new protocol). Briefly, a 4F or 5F heparin-coated catheter was introduced into the proper hepatic artery. Cisplatin (60 mg/m2) dissolved in 100 ml saline for 10 min followed by 5-FU (600 mg/m2) in 100 ml saline for 10 min were injected into the proper and more distant hepatic arteries, respectively. Then, mitomycin C (3 mg/m2) dissolved in 3 to 5 ml of saline mixed with 3 to 5 ml of degradable starch microspheres (DSMs) (Spherex; Yakult, Tokyo, Japan) was administered. This procedure was repeated twice at 4 weeks after surgery with a 1-month interval.
Follow-up
The follow-up program included serum α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonists-II (PIVKA-II) assays every 1 to 2 months. Imaging follow-up was performed using transabdominal ultrasonography (US) examination or computed tomography (CT) every 3 to 4 months. Magnetic resonance imaging (MRI) was performed when deemed necessary. During follow-up, if recurrence was recognized the patient was treated with a second hepatectomy, TACE, radiofrequency ablation, radiotherapy, or chemotherapy.
Data collection
Information on 15 variables was collected for each patient as potential risk factors for recurrence and predictors of survival. The extents of vascular involvement and tumor differentiation were confirmed in the resected specimen. Segmentectomy of ≥3 was defined as a major hepatic resection. Others were defined as minor hepatic resections. The continuous variables of age, indocyanine green retention at 15 min (ICG-R15), main tumor size, and serum AFP and PIVKA-II levels were categorized by cutoff values according to their median values: 64 years, 10 %, 60 mm, 70.2 ng/ml, and 697 IU/ml, respectively.
Statistical analyses
Student’s t test, the χ2 test,or Fisher’s exact test was used where appropriate to compare the clinical and histologic parameters between the two groups. The cumulative survival curves were obtained using the Kaplan–Meier method. Survival curves were statistically compared using the log-rank test. Univariate analysis of the data from all cases was performed. Variables that exhibited statistical significance in the univariate analysis were subsequently included in the multivariate analysis, which was performed using Cox proportional hazard analysis. For all tests, the level of significance was set at p < 0.05.
Results
The clinical and pathological characteristics of the patients are summarized in Tables 1 and 2. The mean age was significantly lower in the HAI group than in the non-HAI group. The rate of poor differentiation was significantly higher in the HAI group than in the non-HAI group. However, there were no significant differences between the HAI and non-HAI groups with respect to any other variable. The surgical parameters including operative procedure, operative duration, and blood infusion rates, were comparable between the two groups.
Compliance and side effects of chemotherapy and hepatic resection
A total of 35 patients (92 %) completed HAI after hepatic resection. The side effects of adjuvant HAI (according to CTCAE v4.0) are shown in Table 3 .HAI was stopped because of tumor recurrence, grade 4 neutropenia, and therapy refusal in one patient each. No lethal side effects of HAI were observed. Five patients (13 %) experienced grade 3/4 adverse events. Three patients had grade 3 vomiting, and three had severe neutropenia (one with grade 4, two with grade 3). One patient (4.1 %) developed a hepatic arterial occlusion caused by the implanted catheter. Among all 73 patients, postoperative complications occurred in 21 (28.7 %): significant pleural effusion or ascites in 7, bile leakage in 3, surgical-site infection in 3, liver failure in 2, abdominal abscess in 2, intraabdominal bleeding in 1, duodenal ulcer in 1, wound dehiscence in 1, and cholangitis in 1.
Tumor recurrence
At a median follow-up of 25 months (range 3–149 months), 23 (60.5 %) and 29 (82.8 %) patients in the HAI and non-HAI groups, respectively, developed tumor recurrence. The rate of recurrence in the remnant liver in the HAI group was 31.5 %, which was significantly lower than 68.5 % in the non-HAI group (p = 0.001). Extrahepatic recurrence rates were not significantly different between the two groups (p = 0.360). In all, 45 patients—20 (52.6 %) in the HAI group and 25 (71.4 %) in the non-HAI group—developed recurrence within 2 years after hepatic resection, but the recurrence rates within 2 years were similar between the two groups (p = 0.098).
Disease-free and overall survival
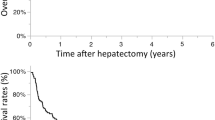
There was no significant difference in recurrence or survival rates between the patients who received HAI according to the old (n = 25) and new (n = 13) protocols: 3-year disease-free survival (DFS) 55.0 % vs. 50.0 %; 3-year overall survival (OS), 53.0 % vs. 54.2 %). Both the 3- and 5-year DFS rates were 33.1 % for patients treated with HAI and 11.8 % for patients in the non-HAI group. The HAI group achieved significantly better outcomes than the non-HAI group (p = 0.029) (Fig. 1a). The 3- and 5-year OS rates were 56.2 % and 46.7 % in the HAI group and 37.4 % and 32.7 % in the non-HAI group, respectively (p = 0.318) (Fig. 1b).
a Disease-free survival (DFS) curves after hepatic resection with or without hepatic arterial infusion (HAI) chemotherapy in 73 patients. The HAI group (solid line) exhibited significantly better DFS than the non-HAI group (dashed line) (p = 0.029). b Overall survival (OS) curves after hepatic resection in the HAI (solid line) and the non-HAI groups (dashed line) in 73 patients. There was no significant difference between the two groups with respect to OS (p = 0.318)
Univariate analysis for DFS and OS was performed for the 73 patients with macroscopic vascular invasion. Tumor size, number of tumors, International Union Against Cancer stage (UICC, 7th edition), and HAI were recognized as potential prognostic factors for DFS (Table 4). AFP, tumor size, and the degree of vascular invasion were recognized as potential prognostic factors for OS (Table 5). Multivariate analysis revealed that the number of tumors [hazard ratio (HR) and 95 % confidence interval (CI): 2.246 (1.158–4.359)] and HAI [HR and 95 % CI): 0.536 (0.306–0.940)] were independent factors for DFS (Table 6). Only tumor size of ≥60 mm was an independent prognostic factor for OS in the multivariate analysis ratio [HR and 95 % CI: 2.296 (1.106–4.767); p = 0.025].
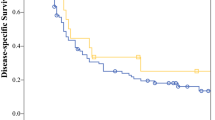
On the other hand, among the limited patients with Vp3/4 or Vv3 (n = 32), the 3-year DFS and OS rates were 33.7 % and 56.8 % in the HAI group and 8.3 % and 12.0 % in the non-HAI group, respectively (Fig. 2). The HAI group had significantly better DFS and OS than the non-HAI group (p = 0.023 and 0.049, respectively). However, among patients with Vp2 or Vv2, the 3-year DFS and OS rates were 32.6 % and 57.0 % in the HAI group and 13.7 % and 52.0 % in the non-HAI group, respectively. There were no significant differences between these two groups.
a DFS curves after hepatic resection with or without HAI for the patients with Vp3/4 or Vv3 (n = 32). The HAI group (solid line) showed significantly better DFS than the non-HAI group (dashed line) (p = 0.023). b OS curves after hepatic resection with or without HAI for the patients with Vp3/4 or Vv3 (n = 32). The HAI group (solid line) showed significantly better OS than the non-HAI group (dashed line) (p = 0.049)
Discussion
The prognosis of HCC patients with apparent PVTT is limited to a few months after diagnosis [8, 17–19]. Although hepatic resection as a monotherapy demonstrates relatively favorable 5-year survival rates (4.0–28.5 %) and median survival times (6–14 months), the recurrence rates are extremely high [20–24]. Portal venous invasion is the most significant risk factor for the early postoperative recurrence of HCC [25]. Therefore, other novel strategies to prevent recurrence are required. At present, combined treatment consisting of hepatic resection and TACE or HAI shows the most promising results. The 5-year survival rate and median survival time for TACE followed by hepatic resection are 42 % and 31 months, respectively [26]. For hepatic resection followed by HAI, these numbers are 36 % and 22 months. respectively [11].
Accordingly, in 1997 we initially started the protocol of arterial infusion of cisplatin (total 150 mg) followed by 5-FU (total 3750 mg). The rationale behind this treatment regimen is that cisplatin and 5-FU have an antitumor effect. Moreover, cisplatin has a synergistic effect as a modulator for 5-FU, and cisplatin and 5-FU can be administered at low doses to reduce adverse reactions. The treatment duration of our protocol—three courses of low-dose cisplatin plus 5-FU—is shorter than that reported in the literature. However, it may be sufficient to reduce the risk of recurrence because of the favorable prognosis of our treatment. The 3-year DFS and OS were 55.0 % and 53.0 %, respectively, which are comparable to those reported in the literature [27–29]. Nevertheless, one patient developed hepatic arterial occlusion caused by the implanted catheter.
Based on this experience, in 2007 we initiated a new protocol without an injection port: two rounds of one-shot HAI consisting of cisplatin (60 mg/mm2 per 10 min), 5-FU (60 mg/mm2 per 10 min) followed by mitomycin-C (3 mg/mm2) with DSM. In the new protocol, we used arterial administration using DSM and mitomycin-C. DSM is a micro-embolic material made from potato starch, 45 ± 7 × 10−6 mm in diameter, that induces transient occlusion of small arteries for 1 h [30]. Co-administration of an anticancer drug with DSM reduces drug dilution because of hepatic arterial flow and enhances drug retention in tumors better than with a single bolus injection [30]. We adopted DSM rather than permanent embolic materials to decrease the damage to the remnant liver during the early postoperative period (4–8 weeks after surgery).
A retrospective case–control study of 127 HCC patients demonstrated that adjuvant chemolipiodolization following hepatic resection is an independent prognostic factor for 2-year recurrence-free survival [HR and 95 % CI: 0.55 (0.34–0.90); p = 0.02] compared to hepatic resection alone [3]. However, the ratio of vascular involvement patients in that study was only 19 % [3]. In a randomized controlled manner, 126 HCC patients with PVTT were divided into control and TACE groups [27]. The control group underwent surgery and PVTT removal, and the TACE group underwent the same procedure combined with postoperative adjuvant TACE. The median survival time of 13 months and a 5-year survival rate of 21.5 % in the TACE group were significantly greater than the corresponding values of 9 months and 8.5 % in the control group [27]. The treatment was started 3 to 4 weeks after surgery and was repeated once every 1 to 2 months for two to five courses (mean 1.8). This postoperative adjuvant TACE resulted in significantly greater OS. The starting point and total course of adjuvant therapy were similar to those of our new protocol during the late period (beginning at 4 weeks for a total of two courses).
Despite the greater number of patients with poorly differentiated HCCs, which is associated with an extremely high recurrence rate [31], the HAI group exhibited significantly better DFS than the non-HAI group. Furthermore, multivariate analysis revealed that the application of HAI is an independent favorable prognostic factor for DFS (Fig. 1a, Table 6). On the other hand, analysis of all patients revealed comparable extrahepatic recurrence rates in the two groups and that HAI was not a favorable prognostic factor for OS. Therefore, some systemic chemotherapy in addition to the HAI therapy might be required to reduce the risk of extrahepatic recurrence, ultimately increasing the OS. Because intrahepatic metastases after hepatic resection are reported to occur usually within 2 years postoperatively [32, 33] and PVTT often causes extensive intrahepatic metastasis of the tumor through the portal tract, the primary objective of adjuvant HAI is to reduce the risk of intrahepatic metastasis via the portal vein rather than multicentric recurrence [34]. Our results did not indicate any obvious differences between the two groups with respect to the recurrence rate within 2 years. However, the overall recurrence rate of the remnant liver in the HAI group was 31.5 %, which was significantly lower than the 68.5 % in the non-HAI group (p = 0.001).
In our analysis, the HAI group demonstrated significantly better DFS and OS than the non-HAI group in the limited Vp3/4 or Vv3 patients (Fig. 2) but not in the Vp2 or Vv2 patients. There is little information regarding recurrence or long-term survival with respect to the degree of tumor thrombi. Vp2 or Vv2 patients may not have widespread dissemination of tumor cells via the portal or hepatic vein. Furthermore, the extent of resected liver is limited, and the removal of tumor thrombi is easier and more complete than in Vp3/4 and Vv3 patients. In fact, operating time, blood loss, and the frequency of blood transfusion were significantly smaller in Vp2 and Vv2 patients (data not shown).
PVTT with multinodular or diffuse recurrence (i.e., intrahepatic dissemination) of HCC after thermal ablation is one of the most serious problems resulting in a poor prognosis [10]. In this study, seven patients exhibited this recurrence pattern according to the histopathologic examinations of the resected specimens, and five patients underwent adjuvant HAI. The 5-year OS and DFS rates of these five patients were 80 % and 40 %, respectively, suggesting that hepatic resection followed by adjuvant HAI is also useful for PVTT with intrahepatic dissemination if the recurrence site is localized in the liver.
We applied mono- or bi-sectionectomy or (extended) hemi-hepatectomy in 42 (93.3 %) HCC patients with grade Vp2 or Vv2. Tumor thrombi might be completely removed by performing surgery alone in such cases. Among the nine patients with Vp3/4 (32.1 %), we applied the peeling-off technique for those with massive PVTTs beyond the bifurcation or into other sectors. This method is reported to have no disadvantages regarding curability compared to an en bloc technique [15]. The favorable 3-year DFS (32.4 %) and OS (58.3 %) rates of these nine patients may demonstrate the efficacy of the peeling-off technique for patients with grade Vp3/4.
Multivariate analysis revealed that adjuvant HAI was one of the independent favorable prognostic factors for DFS. However, the main limitation of this study is that it was not a randomized prospective study. Therefore, some confounders might have affected the results. The patients diagnosed with early recurrence (within 1 month) were analyzed as the non-HAI group because we aimed to investigate the advantages of adjuvant HAI in patients with resectable HCC and if HAI could be completed within 1 to 2 months. However, with the entry criteria used for the non-HAI group (early recurrence, advanced age, co-morbidities, poor performance), there is clearly a selection bias with less favorable prognostic factors in these patients.
Conclusions
Adjuvant HAI for HCC patients with macroscopic vascular invasion, especially patients with Vp3/4 or Vv3, might reduce the risk of recurrence without serious complications. However, a prospective randomized study is required to confirm our findings.
References
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340:745–750
Poon D, Anderson BO, Chen LT et al (2009) Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 10:1111–1118
Ueno M, Uchiyama K, Ozawa S et al (2011) Adjuvant chemolipiodolization reduces early recurrence derived from intrahepatic metastasis of hepatocellular carcinoma after hepatectomy. Ann Surg Oncol 8:3624–3631
Imamura H, Matsuyama Y, Tanaka E et al (2003) Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 38:200–207
Llovet JM, Fuster J, Bruix J (1999) Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 30:1434–1440
Poon RT, Fan ST, Ng IO et al (2000) Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 89:500–507
Ando E, Yamashita F, Tanaka M et al (1997) A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer 79:1890–1896
Okuda K, Ohtsuki T, Obata H et al (1985) Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer 56:918–928
Pirisi M, Avellini C, Fabris C et al (1998) Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin 124:397–400
Masuda T, Beppu T, Ishiko T et al (2008) Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg 15:589–595
Fukuda S, Okuda K, Imamura M et al (2002) Surgical resection combined with chemotherapy for advanced hepatocellular carcinoma with tumor thrombus: report of 19 cases. Surgery 131:300–310
Ando E, Tanaka M, Yamashita F et al (2002) Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein: analysis of 48 cases. Cancer 95:588–595
Sakon M, Nagano H, Dono K et al (2002) Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer 94:435–442
Beppu T, Ohara C, Yamaguchi Y et al (1991) A new approach to hemoembolization for unresectable hepatocellular carcinoma using aclarubicin microspheres in combination with cisplatin suspended in iodized oil. Cancer 68:2555–2560
Beppu T, Ishiko T, Chikamoto A et al (2012) Liver hanging maneuver decreases blood loss and operative time in a right-side hepatectomy. Hepatogastroenterology (in press)
Inoue Y, Hasegawa K, Ishizawa T et al (2009) Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery 145:9–19
The Liver Cancer Study Group of Japan (1994) Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer 74:2772–2780
Cady B (1983) Natural history of primary and secondary tumors of the liver. Semin Oncol 10:127–134
Llovet JM, Bustamante J, Castells A et al (1999) Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 29:62–67
Minagawa M, Makuuchi M (2006) Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 21:7561–7567
Ikai I, Yamaoka Y, Yamamoto Y et al (1998) Surgical intervention for patients with stage IV-A hepatocellular carcinoma without lymph node metastasis: proposal as a standard therapy. Ann Surg 227:433–439
Wu CC, Hsieh SR, Chen JT et al (2000) An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg 135:1273–1279
Le Treut YP, Hardwigsen J, Ananian P et al (2006) Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature: a European case-control series. J Gastrointest Surg 10:855–862
Zhou J, Tang ZY, Wu ZQ et al (2006) Factors influencing survival in hepatocellular carcinoma patients with macroscopic portal vein tumor thrombosis after surgery, with special reference to time dependency: a single-center experience of 381 cases. Hepatogastroenterology 53:275–280
Choi KK, Kim SH, Choi SB et al (2011) Portal venous invasion: the single most independent risk factor for immediate postoperative recurrence of hepatocellular carcinoma. J Gastroenterol Hepatol 26:1646–1651
Minagawa M, Makuuchi M, Takayama T et al (2001) Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg 233:379–384
Peng BG, He Q, Li JP et al (2009) Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg 198:313–318
Niguma T, Muira T, Tutui N (2005) Adjuvant arterial infusion chemotherapy after resection of hepatocellular carcinoma with portal thrombosis: a pilot study. J Hepatobilliary Pancreat Surg 12:249–253
Huang YH, Wu JC, Lui WY et al (2000) Prospective case-controlled trial of adjuvant chemotherapy after resection of hepatocellular carcinoma. World J Surg 24:551–555. doi:10.1007/s002689910090
Ishida K, Hirooka M, Hiraoka A et al (2008) Treatment of hepatocellular carcinoma using arterial chemoembolization with degradable starch microspheres and continuous arterial infusion of 5-fluorouracil. Jpn J Clin Oncol 38:596–603
Ikeda K, Saitoh S, Tsubota A et al (1993) Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer 71:19–25
Portolani N, Coniglio A, Ghidoni S et al (2006) Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 243:229–235
Sakon M, Umeshita K, Nagano H et al (2000) Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg 135:1456–1459
Yamanaka N, Okamoto E, Fujihara S et al (1992) Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer 70:2263–2267
Ichida F, Tsuji T, Omata M (1996) New Inuyama classification; new criteria for histological assessment of chronic hepatitis. Int Hepatol Commun 6:112–119
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nitta, H., Beppu, T., Imai, K. et al. Adjuvant Hepatic Arterial Infusion Chemotherapy after Hepatic Resection of Hepatocellular Carcinoma With Macroscopic Vascular Invasion. World J Surg 37, 1034–1042 (2013). https://doi.org/10.1007/s00268-013-1957-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-1957-1