Abstract
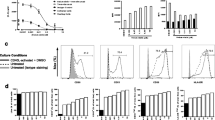
We studied the impact of natural killer T (NKT) cell activation by alpha-galactocysylceramide (α-GalCer, α-GC) on cancer cell repopulation during chemotherapy in murine mesothelioma. The number of NKT cells was found to be increased during the development of murine mesothelioma. NKT cells specifically recognize α-GC through CD1d resulting in their activation and expansion. Tumor-bearing mice were treated with chemotherapy once weekly, and α-GC was followed after each cycle of chemotherapy. Anti-tumor effect was evaluated on wild-type (WT) and CD1d knockout (CD1dKO) mice. Cancer cell proliferation and apoptosis were evaluated by Ki67 and TUNEL immunohistochemistry. CD4+ and CD8+ T cell proportion and activation in tumor, spleen, draining lymph node and peripheral blood were determined by flow cytometry, and gene expression of activated T cell-related cytokines was quantified by reverse transcription PCR. NKT cells were identified by CD1d-α-GC-tetramer staining. In WT mice, tumor growth delay was achieved by cisplatin (Cis), and this effect was improved in combination with α-GC, but α-GC alone had little effect. Cancer cell proliferation during chemotherapy was significantly inhibited by α-GC, while cancer cell death was significantly upregulated. α-GC following chemotherapy resulted in NKT cell expansion and an increase of interferon-γ production in the draining lymph node, blood and spleen. Gene expression of immune-associated cytokines was upregulated. Strikingly, the percentage of inducible T cell co-stimulator+CD4 T cells, Th17/Tc17 cells increased in splenocytes. In CD1d KO mice, however, Cis alone was less effective and Cis + α-GC provided no additional benefit over Cis alone. α-GC alone had minimal effect in both mice. NKT activation between cycles of chemotherapy could improve the outcome of mesothelioma treatment.






Similar content being viewed by others
Abbreviations
- α-GC:
-
α-GalCer, alpha-galactocysylceramide
- APC:
-
Antigen-presenting cell
- Cis:
-
Cisplatin
- DC:
-
Dendritic cell
- ICOS:
-
Inducible T cell co-stimulator
- IFN:
-
Interferon
- KO:
-
Knockout
- MPM:
-
Malignant pleural mesothelioma
- NKT:
-
Natural killer T cell
- sc:
-
Subcutaneously
- WT:
-
Wild type
References
Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM et al (2013) Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339:543–548
Telleria CM (2013) Repopulation of ovarian cancer cells after chemotherapy. Cancer Growth Metastasis 6:15–21
Kim JJ, Tannock IF (2005) Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 5:516–525
Gamarra-Luques CD, Goyeneche AA, Hapon MB, Telleria CM (2012) Mifepristone prevents repopulation of ovarian cancer cells escaping cisplatin–paclitaxel therapy. BMC Cancer 12:200
Wu L, Birle DC, Tannock IF (2005) Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res 65:2825–2831
Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M (2012) CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther 11:1809–1819
Stayner L, Welch LS, Lemen R (2013) The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 34:205–216
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
de Perrot M, Feld R, Cho BC, Bezjak A, Anraku M, Burkes R et al (2009) Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 27:1413–1418
Pasello G, Ceresoli GL, Favaretto A (2013) An overview of neoadjuvant chemotherapy in the multimodality treatment of malignant pleural mesothelioma. Cancer Treat Rev 39:10–17
Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B et al (2001) Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol 167:3114–3122
Tagawa T, Wu L, Anraku M, Yun Z, Rey-McIntyre K, de Perrot M (2013) Antitumor impact of interferon-gamma producing CD1d-restricted NKT cells in murine malignant mesothelioma. J Immunother 36:391–399
Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M et al (2005) Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med 201:1503–1517
Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M et al (2005) A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 11:1910–1917
Godfrey DI, Stankovic S, Baxter AG (2010) Raising the NKT cell family. Nat Immunol 11:197–206
Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y et al (1999) The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med 189:1121–1128
Carnaud C, Gombert J, Donnars O, Garchon H, Herbelin A (2001) Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J Immunol 166:2404–2411
Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R et al (2001) Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol 166:6578–6584
Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P (2009) NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood 113:370–376
Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S et al (2008) NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA 105:8339–8344
Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ et al (2011) Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol 140:130–141
Motohashi S, Okamoto Y, Yoshino I, Nakayama T (2011) Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol 140:167–176
Wu L, Yun Z, Tagawa T, Rey-McIntyre K, Anraku M, de Perrot M (2011) Tumor cell repopulation between cycles of chemotherapy is inhibited by regulatory T-cell depletion in a murine mesothelioma model. J Thorac Oncol 6:1578–1586
Barbieri PG, Marinaccio A, Ferrante P, Scarselli A, Pinelli V, Tassi G (2012) Effects of combined therapies on the survival of pleural mesothelioma patients treated in Brescia, 1982–2006. Tumori 98:215–219
Kelly RJ, Sharon E, Hassan R (2011) Chemotherapy and targeted therapies for unresectable malignant mesothelioma. Lung Cancer 73:256–263
Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL et al (2010) Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 70:9053–9061
Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R et al (2013) Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 121:423–430
Nicol AJ, Tazbirkova A, Nieda M (2011) Comparison of clinical and immunological effects of intravenous and intradermal administration of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin Cancer Res 17:5140–5151
Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H et al (2011) Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol 138:255–265
Peng Y, Zhao L, Shekhar S, Liu L, Wang H, Chen Q et al (2012) The glycolipid exoantigen derived from Chlamydia muridarum activates invariant natural killer T cells. Cell Mol Immunol 9:361–366
Jahn T, Zuther M, Friedrichs B, Heuser C, Guhlke S, Abken H et al (2012) An IL12-IL2-antibody fusion protein targeting Hodgkin’s lymphoma cells potentiates activation of NK and T cells for an anti-tumor attack. PLoS One 7:e44482
Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E et al (1998) Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res 58:1202–1207
Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG et al (2005) Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 202:1279–1288
Sere K, Felker P, Hieronymus T, Zenke M (2013) TGFbeta1 microenvironment determines dendritic cell development. Oncoimmunology 2:e23083
Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA et al (2011) T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity 35:123–134
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S et al (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31:787–798
Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W et al (2010) Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood 115:5385–5392
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S et al (2009) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141–1149
Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E et al (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 190:1873–1881
Hamai A, Pignon P, Raimbaud I, Duperrier-Amouriaux K, Senellart H, Hiret S et al (2012) Human T(H)17 immune cells specific for the tumor antigen MAGE-A3 convert to IFN-gamma-secreting cells as they differentiate into effector T cells in vivo. Cancer Res 72:1059–1063
Caminschi I, Venetsanakos E, Leong CC, Garlepp MJ, Scott B, Robinson BW (1998) Interleukin-12 induces an effective antitumor response in malignant mesothelioma. Am J Respir Cell Mol Biol 19:738–746
Jackaman C, Nelson DJ (2010) Cytokine-armed vaccinia virus infects the mesothelioma tumor microenvironment to overcome immune tolerance and mediate tumor resolution. Cancer Gene Ther 17:429–440
Schmieg J, Yang G, Franck RW, Tsuji M (2003) Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med 198:1631–1641
Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N et al (2011) Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 208:1163–1177
Brennan PJ, Brigl M, Brenner MB (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13:101–117
Mattarollo SR, Smyth MJ (2013) NKT cell adjuvants in therapeutic vaccines against hematological cancers. Oncoimmunology 2:e22615
Acknowledgments
This work was partly supported by the Mesothelioma Foundation at Princess Margaret Hospital, Canada, and the research grant from the Mesothelioma Applied Research Foundation, USA. Dr. Marc de Perrot is the recipient of the grants and the Head of Toronto Mesothelioma Research Program, Canada. We would like to thank the Flow Cytometry Facility of the Hospital for Sick Children and Animal Resources Centre, University Health Network, Toronto, Canada.
Conflict of interest
No potential conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Some preliminary results were presented at the 15th World Conference on Lung Cancer (WCLC2013), Sydney, Australia (abstract published in the Journal of Thoracic Oncology, Volume 8, Supplement 2, November 2013).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, L., Yun, Z., Tagawa, T. et al. Activation of CD1d-restricted natural killer T cells can inhibit cancer cell proliferation during chemotherapy by promoting the immune responses in murine mesothelioma. Cancer Immunol Immunother 63, 1285–1296 (2014). https://doi.org/10.1007/s00262-014-1597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1597-9