Abstract
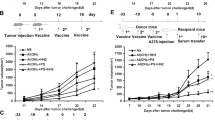
Previous studies have shown that there are profuse lymphatic tissues under the intestinal mucous membrane. Moreover, vaccine administered orally can elicit both mucous membrane and system immune response simultaneously, accordingly induce tumor-specific cytotoxic T lymphocyte. As a result, the oral route is constituted the preferred immune route for vaccine delivery theoretically. However, numerous vaccines especially protein/peptide vaccines remain poorly available when administered by this route. Nanoemulsion has been shown as a useful vehicle can be developed to enhance the antitumor immune response against antigens encapsulated in it and it is good for the different administration routes. Of particular interest is whether the protein vaccine following peroral route using nanoemulsion as delivery carrier can induce the same, so much as stronger antitumor immune response to following conventional ways such as subcutaneous (sc.) or not. Hence, in the present study, we encapsulated the MAGE1-HSP70 and SEA complex protein in nanoemulsion as nanovaccine NE (MHS) using magnetic ultrasound method. We then immuned C57BL/6 mice with NE (MHS), MHS alone or NE (-) via po. or sc. route and detected the cellular immunocompetence by using ELISpot assay and LDH release assay. The therapeutic and tumor challenge assay were examined then. The results showed that compared with vaccination with MHS or NE (-), the cellular immune responses against MAGE-1 could be elicited fiercely by vaccination with NE (MHS) nanoemulsion. Furthermore, encapsulating MHS in nanoemulsion could delay tumor growth and defer tumor occurrence of mice challenged with B16-MAGE-1 tumor cells. Especially, the peroral administration of NE (MHS) could induce approximately similar antitumor immune responses to the sc. administration, but the MHS unencapsulated with nanoemulsion via po. could induce significantly weaker antitumor immune responses than that via sc., suggesting nanoemulsion as a promising carrier can exert potent antitumor immunity against antigen encapsulated in it and make the tumor protein vaccine immunizing via po. route feasible and effective. It may have a broad application in tumor protein vaccine.





Similar content being viewed by others
Abbreviations
- APCs:
-
Antigen-presenting cells
- CTL:
-
Cytotoxic T lymphocyte
- HLA:
-
Human leukocyte antigen
- HPLC:
-
High performance liquid chromatography
- HSP:
-
Heat shock protein
- IFN-γ:
-
Interferon-γ
- MAGE:
-
Melanoma associated antigen
- MHS:
-
MAGE1-HSP70/SEA complex protein
- NE:
-
Nanoemulsion
- NE (-):
-
Nanoemulsion-encaupulated nothing
- NE (MHS):
-
Nanoemulsion-encaupsulated (MAGE1-HSP70/SEA complex protein)
- po.:
-
Peroral
- sc.:
-
Subcutaneous
- SEA:
-
Staphylococcal enterotoxins A
- TEM:
-
Transmission electron microscopy
- TSA:
-
Tumor-specific antigen
References
Alpar HO, Field WN, Hayes K, Lewis DA (1989) A possible use of orally administered microspheres in the treatment of inflammation. J Pharm Pharmacol 41(Suppl):50
Ammoury N, Fessi H, Devissaguet JP, Dubrasquet M, Benita S (1991) Jejunal absorption, pharmacological activity, and pharmacokinetic evaluation of indomethacin-loaded poly(d,l-lactide) and poly(isobutyl-cyanoacrylate) nanocapsules in rats. Pharm Res 8:101
Beck PH, Kreuter J, Müller WEG, Schatton W (1994) Improved peroral delivery of avarol with polyalkylcyanoacrylate nanoparticles. Eur J Pharm Biopharm 40:134
Bonduelle S, Carrier M, Pimienta C, Benoit JP, Lenaerts V (1996) Tissue concentration of nanoencapsulated radiolabelled cyclosporin following peroral delivery in mice or ophthalmic applications in rabbits. Eur J Pharm Biopharm 42:313
Challacombe SJ, Rahman D, O’Hagan DT (1997) Salivary, gut, vaginal and nasal antibody responses after oral immunization with biodegradable microparticles. Vaccine 15:169
Constantinides PP, Lancaster CM, Marcello J, Chiossone DC, Orner D, Hidalgo I, Smith PL, Sarkahian AB, Yiv SH, Owen AJ (1995) Enhanced intestinal absorption of an RGD peptide from water-in-oil microemulsions of different composition and particle size. J Control Rel 34:109
Constantinides PP (1995) Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res 12:1561
Constantinides PP, Scalart JP, Lancaster S, Marcello J, Marks G, Ellens H, Smith PL (1994) Formulation and intestinal absorption enhancement evaluation of water-in-oil microemulsions incorporating medium-chain glycerides. Pharm Res 11:1385
Damgé C, Aprahamian M, Balboni G, Hoeltzel A, Andrieu V, Devissaguet JP (1987) Polyalkylcyanoacrylate nanocapsules increase the intestinal absorption of a lipophilic drug. Int J Pharm 36:121
Damgé C, Michel C, Aprahamian M, Couvreur P, Devissaguet JP (1990) Nanocapsules as carriers for oral peptide delivery. J Control Release 13:233
Damgé C, Michel C, Aprahamian M, Couvreur P (1988) New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes 37:246
Damgé C, Vonderscher J, Marbach P, Pinget M (1997) Poly(alkyl cyanoacrylate) nanocapsules as a delivery system in the rat for octreotide, a long-acting somatostatin analogue. J Pharm Pharmacol 49:949
des Rieux A, Fievez V, Garinot M, YJs Schneider, Préat V (2006) Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 116:1
Devissaguet JP, Fessi H, Ammoury N, Barratt G (1992) Colloidal drug delivery systems for gastrointestinal application, In: Junginger HE (eds) Drug Targeting and Delivery-Concepts in Dosage Form Design, Ellis Horwood, New York, 71
Esparza I, Kissel T (1992) Parameters affecting the immunogenicity of microencapsulated tetanus toxoid. Vaccine 10:714
Fleischer B, Schrezenmeier H (1988) T cell stimulation by staphylococcal enterotoxins Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med 167:1697
Galindo-Rodriguez SA, Allemann E, Fessi H, Doelker E (2005) Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. Crit Rev Ther Drug Carr Syst 22:419
Ge W, Sui YF, Wu DC, Sun YJ, Chen GS, Li ZS, Si SY, Hu PZ, Huang Y, Zhang XM (2006) MAGE-1/Heat shock protein 70/MAGE-3 fusion protein vaccine in nanoemulsion enhances cellular and humoral immune responses to MAGE-1 or MAGE-3 in vivo. Cancer Immunol Immunother 55:841
Hamman JH, Enslin GM, Kotzé AF (2005) Oral delivery of peptide drugs: barriers and developments. BioDrugs 19:165
Hubert B, Atkinson J, Guerret M, Hoffman M, Devissaguet JP, Maincent P (1991) The preparation and acute antihypertensive effects of a nanocapsular form of darodipine, a dihydropyridine calcium entry blocker. Pharm Res 8:734
Jores K, Mehnert W, Drechsler M, Bunjies H, Johann C, Mader K (2004) Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Release 95:217
Jung T, Kamm W, Breitenbach A, Hungerer KD, Hundt E, Kissel T (2001) Tetanus toxoid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide): evaluation of antibody response after oral and nasal application in mice. Pharm Res 18:352
Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ (1999) Oral immunization with Helicobacter pylori-loaded poly(d, l-lactide-co-glycolide) nanoparticles. Helicobacter 4:33
Maincent P, Le Verge R, Sado P, Couvreur P, Devissaguet JP (1986) Deposition kinetics and oral bioavailability of vincamine-loaded polyalkyl cyanoacrylate nanoparticles. J Pharm Sci 75:955
Ma JH, Sui YF, Ye J, Huang Ya-Yu, Li Zeng-Shan, Chen Guang-Sheng, Qu Ping, Song Hong-Ping, Zhang Xiu-Min (2005) Heat shock protein 70/MAGE-3 fusion protein vaccine can enhance cellular and humoral immune responses to MAGE-3 in vivo. Cancer Immunol Immunother 54:907
Maloy KJ, Donachie AM, O’Hagan DT, Mowat AM (1994) Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology 81:661
Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, Santos CA, Vijayaraghavan K, Montgomery S, Bassett M, Morell C (1997) Biologically erodible microspheres as potential oral drug delivery systems. Nature 386:410
Melief CJ, Kast WM (1995) T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev 145:167
Mutwiri G, Bowersock TL, Babiuk LA (2005) Microparticles for oral delivery of vaccines. Expert Opin Drug Del 2:791
Niidome T, Huang L (2002) Gene therapy progress and prospects: nonviral vectors. Gene Ther 9:1647
O’Hagan DT (1990) Intestinal translocation of particulates—implications for drug and antigen delivery. Adv Drug Del Rev 5:265
Ottenbrite R, Zhao R, Milstein S (1996) A new oral microsphere drug delivery system. Macromol Symp 101:379
Pappo J, Ermak TH (1989) Uptake and translocation of fluorescent latex particles by rabbit Peyer’s patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol 76:144
Ponchel G, Irache JM (1998) Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Del Rev 34:191
Pouton CW (1997) Formulation of self-emulsifying drug delivery systems. Adv Drug Del Rev 25:47
Sarciaux JM, Acar L, Sado PA (1995) Using microemulsion formulations for drug delivery of therapeutic peptides. Int J Pharm 120:127
Storni T, Kundig TM, Senti G, Johansen P (2005) Immunity in response to particulate antigen-delivery systems. Adv Drug Del Rev 57:333
Streatfield SJ (2006) Mucosal immunization using recombinant plant-based oral vaccines. Methods 38:150
Sudo T, Kuramoto T, Komiya S, Inoue A, Itoh K (1997) Expression of MAGE genes in osteosarcoma. J Orthop Res 15:128
Swenson EC, Curatolo WJ (1992) Intestinal permeability enhancement for proteins, peptides and other polar drugs: mechanisms and potential toxicity. Adv Drug Del Rev 8:39
Swenson ES, Milisen WB, Curatolo W (1994) Intestinal permeability enhancement: efficacy, acute local toxicity and reversibility. Pharm Res 11:1132
Torres BA, Kominsky SL, Perrin GQ, Hobeika AC, Johnson HM (2001) Superantigens: the good, the bad, and the ugly. Exp Biol Med 226:164
Vinogradov SV, Bronich TK, Kabanov AV (2002) Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv Drug Del Rev 54:135
Vyas SP, Gupta PN (2007) Implication of nanoparticles/microparticles in mucosal vaccine delivery. Expert Rev Vaccines 6:401
Ye J, Chen GS, Song HP, Li ZS, Huang YY, Qu P, Sun YJ, Zhang XM, Sui YF (2004) Heat shock protein 70/MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo. Cancer Immunol Immunother 53:825
Acknowledgments
This work was supported by grants from China National Natural Science Foundation (No. 30271464, No. 30700994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, W., Li, Y., Li, ZS. et al. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following peroral administration route. Cancer Immunol Immunother 58, 201–208 (2009). https://doi.org/10.1007/s00262-008-0539-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0539-9