Abstract
Purpose
The disease course of multiple sclerosis (MS) is unpredictable, and reliable prognostic biomarkers are needed. Positron emission tomography (PET) with β-amyloid tracers is a promising tool for evaluating white matter (WM) damage and repair. Our aim was to investigate amyloid uptake in damaged (DWM) and normal-appearing WM (NAWM) of MS patients, and to evaluate possible correlations between cerebrospinal fluid (CSF) β-amyloid1-42 (Aβ) levels, amyloid tracer uptake, and brain volumes.
Methods
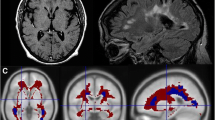
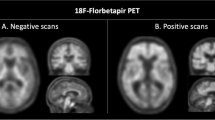
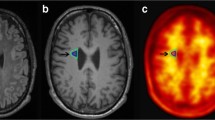
Twelve MS patients were recruited and divided according to their disease activity into active and non-active groups. All participants underwent neurological examination, neuropsychological testing, lumbar puncture, brain magnetic resonance (MRI) imaging, and 18F-florbetapir PET. Aβ levels were determined in CSF samples from all patients. MRI and PET images were co-registered, and mean standardized uptake values (SUV) were calculated for each patient in the NAWM and in the DWM. To calculate brain volumes, brain segmentation was performed using statistical parametric mapping software. Nonparametric statistical analyses for between-group comparisons and regression analyses were conducted.
Results
We found a lower SUV in DWM compared to NAWM (p < 0.001) in all patients. Decreased NAWM-SUV was observed in the active compared to non-active group (p < 0.05). Considering only active patients, NAWM volume correlated with NAWM-SUV (p = 0.01). Interestingly, CSF Aβ concentration was a predictor of both NAWM-SUV (r = 0.79; p = 0.01) and NAWM volume (r = 0.81, p = 0.01).
Conclusions
The correlation between CSF Aβ levels and NAWM-SUV suggests that the predictive role of β-amyloid may be linked to early myelin damage and may reflect disease activity and clinical progression.




Similar content being viewed by others
References
Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med. 2018;378:169–80.
Lassmann H. Multiple sclerosis: Lessons from molecular neuropathology. Exp Neurol. 2014:2–7.
Franklin RJM, Ffrench-Constant C. Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci. 2008:839–55.
Filippi M, Paty DW, Kappos L, Barkhof F, Compston DAS, Thompson AJ, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: A follow-up study. Neurology. 1995;45:255–60.
Stankoff B, Freeman L, Aigrot MS, Chardain A, Dollé F, Williams A, et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-11C]-2-(4-methylaminophenyl)- 6-hydroxybenzothiazole. Ann Neurol. 2011;69:673–80.
Bodini B, Louapre C, Stankoff B. Advanced imaging tools to investigate multiple sclerosis pathology. Presse Med. 2015:e159–e67.
Payoux P. Salabert AS. New PET markers for the diagnosis of dementia. Curr Opin Neurol. 2017:608–16.
Matías-Guiu JA, Oreja-Guevara C, Cabrera-Martín MN, Moreno-Ramos T, Carreras JL, Matías-Guiu J. Amyloid proteins and their role in multiple sclerosis. Considerations in the use of amyloid-PET imaging. Front Neurol. 2016.
Bodini B, Veronese M, García-Lorenzo D, Battaglini M, Poirion E, Chardain A, et al. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann Neurol. 2016;79:726–38.
Matías-Guiu JA, Cabrera-Martín MN, Matías-Guiu J, Oreja-Guevara C, Riola-Parada C, Moreno-Ramos T, et al. Amyloid PET imaging in multiple sclerosis: an 18F-florbetaben study. BMC Neurol. 2015;15:243.
Grecchi E, O’Doherty J, Veronese M, Tsoumpas C, Cook GJ, Turkheimer FE. Multimodal Partial-Volume Correction: Application to 18F-Fluoride PET/CT Bone Metastases Studies. J Nucl Med. 2015;56:1408–14.
Glodzik L, Kuceyeski A, Rusinek H, Tsui W, Mosconi L, Li Y, et al. Reduced glucose uptake and Aβ in brain regions with hyperintensities in connected white matter. Neuroimage. 2014;100:684–91.
Glodzik L, Rusinek H, Li J, Zhou C, Tsui W, Mosconi L, et al. Reduced retention of Pittsburgh compound B in white matter lesions. Eur J Nucl Med Mol Imaging. 2015;42:97–102.
Mangiardi M, Crawford DK, Xia X, Du S, Simon-Freeman R, Voskuhl RR, et al. An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol. 2011;21:263–78.
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal Transection in the Lesions of Multiple Sclerosis. N Engl J Med. 1998;338:278–85.
Gehrmann J, Banati RB, Cuzner ML, Kreutzberg GW, Newcombe J. Amyloid precursor protein (APP) expression in multiple sclerosis lesions. Glia. 1995;15:141–51.
Hu X, Hicks CW, He W, Wong P, MacKlin WB, Trapp BD, et al. BACE1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–5.
Augutis K, Axelsson M, Portelius E, Brinkmalm G, Andreasson U, Gustavsson MK, et al. Cerebrospinal fluid biomarkers of β-amyloid metabolism in multiple sclerosis. Mult Scler J. 2013;19:543–52.
Mattsson N, Axelsson M, Haghighi S, Malmeström C, Wu G, Anckarsäter R, et al. Reduced cerebrospinal fluid BACE1 activity in multiple sclerosis. Mult Scler. 2009;15:448–54.
Mori F, Rossi S, Sancesario G, Codecá C, Mataluni G, Monteleone F, et al. Cognitive and cortical plasticity deficits correlate with altered amyloid-Β CSF levels in multiple sclerosis. Neuropsychopharmacology. 2011;36:559–68.
Gentile A, Mori F, Bernardini S, Centonze D. Role of amyloid-beta CSF levels in cognitive deficit in MS. Clin Chim Acta. 2015;449:23–30.
Pietroboni AM, Schiano Di Cola F, Scarioni M, Fenoglio C, Spanò B, Arighi A, et al. CSF β-amyloid as a putative biomarker of disease progression in multiple sclerosis. Mult Scler. 2017;23:1085–91.
Pietroboni AM, Caprioli M, Carandini T, Scarioni M, Ghezzi L, Arighi A, et al. CSF β-amyloid predicts prognosis in patients with multiple sclerosis. Mult Scler J. 2018:135245851879170.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014:278–86.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33:1444.
Bergamaschi R, Montomoli C, Mallucci G, Lugaresi A, Izquierdo G, Grand’Maison F, et al. BREMSO: A simple score to predict early the natural course of multiple sclerosis. Eur J Neurol. 2015;22:981–9.
Goretti B, Patti F, Cilia S, Mattioli F, Stampatori C, Scarpazza C, et al. The Rao’s Brief Repeatable Battery version B: Normative values with age, education and gender corrections in an Italian population. Neurol Sci. 2014;35:79–82.
Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–83.
Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–11.
Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93.
Acknowledgements
This research was supported by Fondazione Monzino and the Italian Ministry of Health (“Ricerca Corrente” to ES). GGF was supported by the Associazione Italiana Ricerca Alzheimer ONLUS (AIRAlzh Onlus)-COOP Italia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pietroboni, A.M., Carandini, T., Colombi, A. et al. Amyloid PET as a marker of normal-appearing white matter early damage in multiple sclerosis: correlation with CSF β-amyloid levels and brain volumes. Eur J Nucl Med Mol Imaging 46, 280–287 (2019). https://doi.org/10.1007/s00259-018-4182-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4182-1