Abstract
Purpose
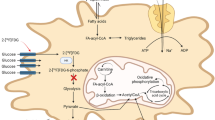
Myocardial inflammation is an emerging target for novel therapies and thus for molecular imaging. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) has been employed, but requires an approach for suppression of cardiomyocyte uptake. We tested clinically viable strategies for their suitability in mouse models in order to optimize preclinical imaging protocols.
Methods
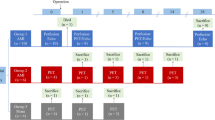
C57BL/6 mice (n = 56) underwent FDG PET under various conditions. In healthy animals, the effect of low-dose (5 units/kg) or high-dose (500 units/kg, 15 min prior) intravenous heparin, extended fasting (18 h) and the impact of conscious injection with limited, late application of isoflurane anaesthesia after 40 min of conscious uptake were examined in comparison to ketamine/xylazine anaesthesia. Conscious injection/uptake strategies were further evaluated at 3 days after permanent coronary artery occlusion.
Results
Under continuous isoflurane anaesthesia, neither heparin administration nor extended fasting significantly impacted myocardial 18F-FDG accumulation. Injection with 40 min uptake in awake mice resulted in a marked reduction of global myocardial 18F-FDG uptake compared to standard isoflurane anaesthesia (5.7 ± 1.1 %ID/g vs 30.2 ± 7.9 %ID/g, p < 0.01). Addition of heparin and fasting further reduced uptake compared to conscious injection alone (3.8 ± 1.5 %ID/g, p < 0.01) similar to ketamine/xylazine (2.4 ± 2.2 %ID/g, p < 0.001). In the inflammatory phase, 3 days after myocardial infarction, conscious injection/uptake with and without heparin/fasting identified a marked increase in myocardial 18F-FDG accumulation that was similar to that observed under ketamine/xylazine.
Conclusion
Continuous isoflurane anaesthesia obscures any suppressive effect of heparin or fasting on cardiomyocyte glucose utilization. Conscious injection of FDG in rodents significantly reduces cardiomyocyte uptake and enables further suppression by heparin and fasting, similar to clinical observations. In contrast to ketamine/xylazine, this represents a more physiological, translatable strategy for suppression of cardiomyocyte 18F-FDG uptake when targeting myocardial inflammation.




Similar content being viewed by others
References
Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J 2013;77:580–7.
Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005;26:1538–43. doi:10.1093/eurheartj/ehi180.
Moon H, Park HE, Kang J, Lee H, Cheong C, Lim YT, et al. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation 2012;125:2603–12. doi:10.1161/CIRCULATIONAHA.111.075283.
Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med 2014;55:99–106. doi:10.2967/jnumed.112.115121.
Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 2012;59:153–63. doi:10.1016/j.jacc.2011.08.066.
Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: a randomized controlled trial. J Nucl Cardiol 2010;17:286–91. doi:10.1007/s12350-009-9179-5.
Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 2009;50:563–8. doi:10.2967/jnumed.108.055616.
Minamimoto R, Morooka M, Kubota K, Ito K, Masuda-Miyata Y, Mitsumoto T, et al. Value of FDG-PET/CT using unfractionated heparin for managing primary cardiac lymphoma and several key findings. J Nucl Cardiol 2011;18:516–20. doi:10.1007/s12350-011-9358-z.
Lee KH, Ko BH, Paik JY, Jung KH, Choe YS, Choi Y, et al. Effects of anesthetic agents and fasting duration on 18F-FDG biodistribution and insulin levels in tumor-bearing mice. J Nucl Med 2005;46:1531–6.
Flores JE, McFarland LM, Vanderbilt A, Ogasawara AK, Williams SP. The effects of anesthetic agent and carrier gas on blood glucose and tissue uptake in mice undergoing dynamic FDG-PET imaging: sevoflurane and isoflurane compared in air and in oxygen. Mol Imaging Biol 2008;10:192–200. doi:10.1007/s11307-008-0137-4.
Thackeray JT, Bankstahl JP, Wang Y, Korf-Klingebiel M, Walte A, Wittneben A, et al. Targeting post-infarct inflammation by PET imaging: comparison of 68Ga-citrate and 68Ga-DOTATATE with 18F-FDG in a mouse model. Eur J Nucl Med Mol Imaging 2014. doi:10.1007/s00259-014-2884-6.
Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 2011;17:581–8. doi:10.1038/nm.2354.
Thorn SL, deKemp RA, Dumouchel T, Klein R, Renaud JM, Wells RG, et al. Repeatable noninvasive measurement of mouse myocardial glucose uptake with 18F-FDG: evaluation of tracer kinetics in a type 1 diabetes model. J Nucl Med 2013;54:1637–44. doi:10.2967/jnumed.112.110114.
Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J 2008;49:17–26.
Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 2006;47:999–1006.
Kreissl MC, Stout DB, Wong KP, Wu HM, Caglayan E, Ladno W, et al. Influence of dietary state and insulin on myocardial, skeletal muscle and brain [F]-fluorodeoxyglucose kinetics in mice. EJNMMI Res 2011;1:8. doi:10.1186/2191-219X-1-8.
Wu HM, Sui G, Lee CC, Prins ML, Ladno W, Lin HD, et al. In vivo quantitation of glucose metabolism in mice using small-animal PET and a microfluidic device. J Nucl Med 2007;48:837–45. doi:10.2967/jnumed.106.038182.
Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Morris DL, Craig JC. Metabolic fate of extracted glucose in normal human myocardium. J Clin Invest 1985;76:1819–27. doi:10.1172/JCI112174.
Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 2011;52:e21–31.
Toyama H, Ichise M, Liow JS, Vines DC, Seneca NM, Modell KJ, et al. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol 2004;31:251–6. doi:10.1016/S0969-8051(03)00124-0.
Wollenweber T, Roentgen P, Schäfer A, Schatka I, Zwadlo C, Brunkhorst T, et al. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ Cardiovasc Imaging 2014;7:811–8. doi:10.1161/CIRCIMAGING.114.001689.
Acknowledgments
The authors thank the preclinical molecular imaging and radiochemistry staff of the Department of Nuclear Medicine for their skilful technical assistance. JTT is supported by fellowships from the German Academic Exchange Service (DAAD) and the Canadian Institutes of Health Research. This project was partially funded by the REBIRTH-2 Cluster of Excellence and by EU FP7 grant PIRG08-GA-2010-276889 (FMB).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thackeray, J.T., Bankstahl, J.P., Wang, Y. et al. Clinically relevant strategies for lowering cardiomyocyte glucose uptake for 18F-FDG imaging of myocardial inflammation in mice. Eur J Nucl Med Mol Imaging 42, 771–780 (2015). https://doi.org/10.1007/s00259-014-2956-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2956-7