Abstract
Purpose
α2C-Adrenoceptors share inhibitory presynaptic functions with the more abundant α2A-adrenoceptor subtype, but they also have widespread postsynaptic modulatory functions in the brain. Research on the noradrenergic system of the human brain has been hampered by the lack of suitable PET tracers targeted to the α2-adrenoceptor subtypes.
Methods
PET imaging with the specific α2C-adrenoceptor antagonist tracer [11C]ORM-13070 was performed twice in six healthy male subjects to investigate the test–retest reliability of tracer binding.
Results
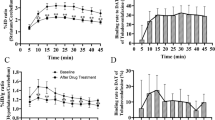
The bound/free ratio of tracer uptake relative to nonspecific uptake into the cerebellum during the time interval of 5 – 30 min was most prominent in the dorsal striatum: 0.77 in the putamen and 0.58 in the caudate nucleus. Absolute test–retest variability in bound/free ratios of tracer ranged from 4.3 % in the putamen to 29 % in the hippocampus. Variability was also <10 % in the caudate nucleus and thalamus. Intraclass correlation coefficients (ICC) ranged from 0.50 in the hippocampus to 0.89 in the thalamus (ICC >0.70 was also reached in the caudate nucleus, putamen, lateral frontal cortex and parietal cortex). The pattern of [11C]ORM-13070 binding, as determined by PET, was in good agreement with receptor density results previously derived from post-mortem autoradiography. PET data analysis results obtained with a compartmental model fit, the simplified reference tissue model and a graphical reference tissue analysis method were convergent with the tissue ratio method.
Conclusion
The results of this study support the use of [11C]ORM-13070 PET in the quantitative assessment of α2C-adrenoceptors in the human brain in vivo. Reliable assessment of specific tracer binding in the dorsal striatum is possible with the help of reference tissue ratios.




Similar content being viewed by others
References
Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–20.
MacDonald E, Kobilka BK, Scheinin M. Gene targeting – homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–9.
Fagerholm V, Rokka J, Nyman L, Sallinen J, Tiihonen J, Tupala E, et al. Autoradiographic characterization of alpha(2C)-adrenoceptors in the human striatum. Synapse. 2008;62:508–15.
Holmberg M, Fagerholm V, Scheinin M. Regional distribution of alpha(2C)-adrenoceptors in brain and spinal cord of control mice and transgenic mice overexpressing the alpha(2C)-subtype: an autoradiographic study with [(3)H]RX821002 and [(3)H]rauwolscine. Neuroscience. 2003;117:875–98.
Scheinin M, Sallinen J, Haapalinna A. Evaluation of the alpha2C-adrenoceptor as a neuropsychiatric drug target studies in transgenic mouse models. Life Sci. 2001;68:2277–85.
Kalkman HO, Loetscher E. alpha2C-Adrenoceptor blockade by clozapine and other antipsychotic drugs. Eur J Pharmacol. 2003;462:33–40.
Rouru J, Wesnes K, Hänninen J, Murphy M, Riordan H, Rinne J. Safety and efficacy of ORM-12741 on cognitive and behavioral symptoms in patients with Alzheimer’s disease: A randomized, double-blind, placebo-controlled, parallel group, multicenter, proof-of-concept 12 week study. AAN 65th Annual Meeting Abstracts, 2013. Abstract 002.
Arponen E, Helin S, Marjamäki P, Grönroos T, Holm P, Löyttyniemi E, et al. A PET tracer for brain alpha2C adrenoceptors, 11C-ORM-13070: radiosynthesis and preclinical evaluation in rats and knockout mice. J Nucl Med. 2014;55:1171–7.
Luoto P, Suilamo S, Oikonen V, Arponen E, Helin S, Herttuainen J, et al. 11C-ORM-13070, a novel PET ligand for brain α2c-adrenoceptors: radiometabolism, plasma pharmacokinetics, whole-body distribution and radiation dosimetry in healthy men. Eur J Nucl Med Mol Imaging. 2014. doi:10.1007/s00259-014-2782-y
Alakurtti K, Aalto S, Johansson JJ, Nagren K, Tuokkola T, Oikonen V, et al. Reproducibility of striatal and thalamic dopamine D2 receptor binding using [11C]raclopride with high-resolution positron emission tomography. J Cereb Blood Flow Metab. 2011;31:155–65.
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8.
Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–70.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Farde L, Eriksson L, Blomquist G, Halldin C. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET – a comparison to the equilibrium analysis. J Cereb Blood Flow Metab. 1989;9:696–708.
Alakurtti K, Johansson JJ, Tuokkola T, Nagren K, Rinne JO. Rostrocaudal gradients of dopamine D2/3 receptor binding in striatal subregions measured with [(11)C]raclopride and high-resolution positron emission tomography. Neuroimage. 2013;82:252–9.
Slifstein M, Hwang DR, Martinez D, Ekelund J, Huang Y, Hackett E, et al. Biodistribution and radiation dosimetry of the dopamine D2 ligand 11C-raclopride determined from human whole-body PET. J Nucl Med. 2006;47:313–9.
Virta JR, Tolvanen T, Någren K, Brück A, Roivainen A, Rinne JO. 1-11C-methyl-4-piperidinyl-N-butyrate radiation dosimetry in humans by dynamic organ-specific evaluation. J Nucl Med. 2008;49:347–53.
Hiura M, Nariai T, Ishii K, Sakata M, Oda K, Toyohara J, et al. Changes in cerebral blood flow during steady-state cycling exercise: a study using oxygen-15-labeled water with PET. J Cereb Blood Flow Metab. 2014;34:389–96.
Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, et al. Effects of a alpha 2C-adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology. 2006;31:1750–6.
Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–78.
Marcus MM, Wiker C, Franberg O, Konradsson-Geuken A, Langlois X, Jardemark K, et al. Adjunctive alpha2-adrenoceptor blockade enhances the antipsychotic-like effect of risperidone and facilitates cortical dopaminergic and glutamatergic, NMDA receptor-mediated transmission. Int J Neuropsychopharmacol. 2010;13:891–903.
Alachkar A, Brotchie JM, Jones OT. Changes in the mRNA levels of alpha2A and alpha2C adrenergic receptors in rat models of Parkinson’s disease and L-DOPA-induced dyskinesia. J Mol Neurosci. 2012;46:145–52.
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–74.
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–7.
Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51.
Conflicts of interest
J.H., A.H., J.R. and J.S. are employees of Orion Pharma. M.K. was at the time of the study an employee of Turku Imanet, a GE Healthcare company. The study was funded by Orion Pharma. M.S. and J.O.R. have contract research relationships with Orion Pharma and GE Healthcare and M.S. has received speaker’s fees from Orion Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehto, J., Virta, J.R., Oikonen, V. et al. Test–retest reliability of 11C-ORM-13070 in PET imaging of α2C-adrenoceptors in vivo in the human brain. Eur J Nucl Med Mol Imaging 42, 120–127 (2015). https://doi.org/10.1007/s00259-014-2899-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2899-z