Abstract
Purpose
To determine the prognostic value of tumor-induced cortical bone destruction at computed tomography (CT) in newly diagnosed diffuse large B-cell lymphoma (DLBCL).
Materials and methods
This retrospective study included 105 patients with newly diagnosed DLBCL who had undergone CT and bone marrow biopsy (BMB) before R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisolone) chemo-immunotherapy. Cox regression analyses were used to determine the associations of cortical bone status at CT (absence vs. presence of tumor-induced cortical bone destruction), BMB findings (negative vs. positive for lymphomatous involvement), and dichotomized National Comprehensive Cancer Network International Prognostic Index (NCCN-IPI) strata (low risk vs. high risk) with progression-free survival (PFS) and overall survival (OS).
Results
Univariate Cox regression analysis indicated that cortical bone status at CT was no significant predictor of either PFS or OS (p = 0.358 and p = 0.560, respectively), whereas BMB findings (p = 0.002 and p = 0.013, respectively) and dichotomized NCCN-IPI risk strata (p = 0.002 and p = 0.003, respectively) were significant predictors of both PFS and OS. In the multivariate Cox proportional hazards model, only the dichotomized NCCN-IPI score was an independent predictive factor of PFS and OS (p = 0.004 and p = 0.003, respectively).
Conclusions
The presence of tumor-induced cortical bone destruction at CT was not found to have any prognostic implications in newly diagnosed DLBCL.
Similar content being viewed by others
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma, making up approximately 30 to 35 % of all cases [1, 2]. Diagnosis of bone marrow involvement is important in DLBCL, because it is an adverse prognostic factor with potential consequences for treatment planning [1–3]. Blind bone marrow biopsy (BMB) of the posterior iliac crest is therefore a standard staging procedure in these patients [4]. 18 F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (FDG-PET/CT) may be used complementary or as a partial alternative to BMB for the detection of bone marrow evaluation in DLBCL [5]. Unfortunately, several recent studies have indicated that, unlike BMB-based bone marrow status, FDG-PET/CT-based bone marrow status is not predictive of progression-free survival (PFS) and overall survival (OS) [6–8]. Although the reason for the lack of prognostic value of FDG-PET/CT in this setting is still unclear, it indicates that focal FDG-avid bone marrow lesions that are presumed to be lymphomatous are clinically irrelevant in this context.
CT, either alone or in combination with FDG-PET, is a compulsory staging procedure in DLBCL [4]. Although CT is not an appropriate method to assess the bone marrow and to detect lymphomatous lesions that are confined to the bone marrow [9], it is the best method to evaluate the cortical bone. CT scans of patients with DLBCL sometimes show tumor-induced cortical bone destruction, either as a result of lymphoma extending from the bone marrow to the bony cortex, or as a result of extramedullary lymphoma growing into the adjacent bony cortex. Although cortical destruction shown at CT indicates that the underlying DLBCL has an aggressive tumor growth, the prognostic implications of this finding are still unclear.
The purpose of this study was to determine the prognostic value of tumor-induced cortical bone destruction at CT in newly diagnosed DLBCL patients who are treated with R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisolone) chemo-immunotherapy.
Materials and methods
Study design and patient population
This retrospective study was approved by the local institutional review board and the requirement for written informed consent was waived. All patients with newly diagnosed DLBCL routinely undergo pretreatment CT at our institution. The hospital’s database (which identifies patients on the basis of their clinical diagnosis) was searched for all patients who were newly diagnosed with DLBCL between January 2004 and December 2013. Study inclusion criteria were: newly diagnosed and histologically proven DLBCL, availability of CT (either full-dose CT or low-dose CT as part of an FDG-PET examination) from skull base to upper thigh, availability of blind bone marrow biopsy (BMB) of the iliac crest with a time interval between CT and BMB <30 days, availability of serum lactate dehydrogenase (LDH) measurement, and treatment with the R-CHOP regimen. Study exclusion criteria were: primary mediastinal DLBCL (which is regarded as a different disease entity), previously treated/relapsed lymphoma, transformed lymphoma, coexistence of another lymphoma subtype in the diagnostic biopsy, another malignancy within the previous 5 years, and start of treatment before CT.
CT acquisition
CT scans were acquired using either a stand-alone 16-detector row CT system (Somatom Sensation 16, Siemens Healthcare, Erlangen, Germany) or a 40-detector row integrated PET/CT system (Biograph 40 TruePoint PET/CT, Siemens Healthcare, Erlangen, Germany). All patients ingested 780 ml of oral contrast agent (750 ml of water mixed with 30 ml of Telebrix Gastro, Guerbet, Gorinchem, The Netherlands), 2 h before image acquisition. Full-dose CT from skull base to upper thigh was performed in the portal venous phase, following intravenous administration of a non-ionic iodinated contrast agent (Omnipaque 300 [GE Healthcare, Eindhoven, the Netherlands] for Somatom Sensation 16 or Xenetix 300, [Guerbet, Gorinchem, The Netherlands] for Biograph 40 TruePoint PET/CT), using the following settings: 120 kV, 90 mA (Somatom Sensation 16) or 60–160 mAs (automatic dose modulation) (Biograph 40 TruePoint PET/CT), pitch of 0.8, 0.5-s (Somatom Sensation 16) or 0.8-s (Biograph 40 TruePoint PET/CT) tube rotation time, and 1.5-mm slice width. Typical CTDIvol values for full-dose CT scanning was around 4.0 mGy for premonitoring of the intravenous bolus, 28.3 mGy for monitoring of the intravenous bolus, 6.5 mGy for neck CT, 7.6 mGy for chest CT, and 13.3 mGy for abdomen CT. A subset of patients only underwent non-intravenous contrast-enhanced low-dose CT as part of their FDG-PET/CT examination, which was acquired with the following settings: 120 kV, 26–30 mAs (automatic dose modulation), pitch of 1.2, 0.8-s tube rotation time, and 1.5-mm slice width. Typical CTDIvol value for low-dose CT scanning was around 1.7 mGy. A smooth reconstruction kernel was used, and CT images were reconstructed to contiguous axial, coronal, and sagittal 5-mm slices.
Image interpretation
A reader with more than 5 years of clinical CT experience (T.C.K) evaluated the CT images for the presence of tumor-induced cortical bone destruction. The reader was blinded to clinical, laboratory, and other imaging findings, and patient outcome. Tumor-induced cortical destruction was defined as non-integrity of the bony cortex with underlying hypodense (lytic) bone marrow changes, with or without associated tumor mass in the surrounding soft tissues (Figs. 1 and 2). Vertebral body collapse was only considered positive for tumor-induced cortical destruction if focal or epidural soft tissue masses and/or involvement of the pedicles was also present [10]. If present, the maximum diameter in any plane of the largest area of cortical bone destruction was measured. The reader also evaluated all CT scans for the presence and extent of nodal and extramedullary extranodal disease. For this purpose, any lymph node measuring more than 10 mm in short-axis diameter in the axial plane was considered positive for lymphoma [11]. Focal areas with abnormal attenuation and masses in extranodal organs were also considered positive for lymphoma, excluding obviously benign lesions such as cysts and hemangiomas. Splenomegaly (splenic index exceeding 725 cm3 [12]) was regarded positive for lymphoma, but hepatomegaly was not. Subsequently, presence of extranodal disease in major organs (bone marrow, central nervous system, liver/gastrointestinal tract or lung) and Ann Arbor stage (stage I-IV) [13] were recorded for subsequent National Comprehensive Cancer Network (NCCN) International Prognostic Index (IPI) scoring [3].
BMB
Unilateral BMB of the posterior iliac crest was acquired by different hematologists and evaluated by different hematopathologists, as part of standard care.
NCCN-IPI
Age, serum LDH levels, presence of extranodal disease in major organs (either bone marrow, central nervous system, liver/gastrointestinal tract or lung involvement), presence of Ann Arbor stage III/IV disease, and Eastern Cooperative Oncology Group (ECOG) performance status score of each patient were recorded at time of diagnosis to calculate an NCCN-IPI score [3]. For the purpose of this study, only histologically confirmed bone marrow involvement, and not imaging-based bone marrow involvement, was used to define stage IV disease and extranodal involvement, as recommended by Zhou et al. [3]. The four NCCN-IPI risk groups (low risk [scores 0–1], low-intermediate risk [scores 2–3], high-intermediate risk [scores 4–5] and high risk [scores 6–8]) were dichotomized into low risk (comprising low and low-intermediate risk patients) and high risk (comprising high-intermediate and high-risk patients) groups.
Patient follow-up
Clinical follow-up and either follow-up stand-alone FDG-PET with stand-alone CT or integrated FDG-PET/CT were used in all patients to determine if and when relapsed or progressive disease had occurred, in line with the Revised Response Criteria for Malignant Lymphoma [14]. PFS was calculated from the date of diagnosis to documented disease relapse/progression or, for patients dying as a result of causes unrelated to DLBCL or the lymphoma treatment, the date of death. For surviving patients who did not experience disease relapse/progression, follow-up was censored at the date the patient was last known to be alive. OS was calculated from the date of diagnosis until death as a result of any cause or, in surviving patients, censored at the date last known to be alive.
Statistical analysis
Associations between cortical bone status at CT (absence vs. presence of tumor-induced cortical bone destruction) and the NCCN-IPI factors categorized age (≤40, 40–60, 60–75, and >75 years), categorized LDH ratio (≤1, 1–3 or >3 upper limit of normal), presence of extranodal disease in major organs (either bone marrow [histologically confirmed], central nervous system, liver/gastrointestinal tract or lung), presence of Ann Arbor stage III/IV disease, and ECOG performance status (≥2) were assessed using Spearman (ρ) (for ordinal NCCN-IPI factors) or Phi (φ) (for binary NCCN-IPI factors) correlation coefficient analyses [15].
PFS and OS were analyzed using the Kaplan–Meier method with log-rank test for comparison of differences [16], according to cortical bone status at CT (absence vs. presence of tumor-induced cortical bone destruction), BMB findings (negative vs. positive for lymphomatous involvement), and dichotomized NCCN-IPI risk strata (low risk vs. high risk). Subsequently, univariate and multivariate Cox regression analyses were performed to determine the influence of cortical bone status at CT, BMB findings, and dichotomized NCCN-IPI risk strata on PFS and OS.
Two-sided p values less than 0.05 were considered to indicate a statistically significant difference. Statistical analyses were executed using MedCalc statistical software version 12.6.0 (Ostend, Belgium).
Results
Patients
A total of 208 patients were newly diagnosed with DLBCL between January 2004 and December 2013. Of these 208 patients, four were excluded because of primary mediastinal DLBCL, 15 were excluded because of transformed lymphoma, 18 were excluded because of coexistence of another lymphoma subtype in the diagnostic biopsy, four were excluded because of another malignancy within the previous 5 years, 23 were excluded because of non-availability of CT from skull base to upper thigh, 22 were excluded because of lack of BMB, four were excluded because of a BMB of poor, non-diagnostic quality, three were excluded because the time interval between CT and BMB exceeded 30 days, and ten were excluded because they received no or another treatment than R-CHOP regimen. Finally, 105 patients (57 men and 48 women, mean age, 63.9 years, age range, 24–87 years) were included. Detailed patient characteristics are shown in Table 1.
Cortical bone status at CT
Tumor-induced cortical bone destruction at CT was present in eight of 105 (7.6 %) patients, and was located in the cranium (n = 1), nasal bone (n = 1), mandible (n = 1), clavicle (n = 1), ribs (n = 1), iliac wings (n = 1) and sacrum (n = 2). An associated tumor mass in the surrounding soft tissues was found in three of these eight (37.5 %) patients. The mean maximum diameter of the largest area of cortical bone destruction in those eight patients was 3.5 cm (range, 2–4.5 cm). Representative examples are shown in Figs. 1 and 2.
BMB
BMB was positive for lymphoma in 19 of 105 (18.1 %) patients. Only 3/19 (16 %) patients had tumor-induced cortical bone destruction.
Correlations between cortical bone status at CT and NCCN-IPI factors
There were no correlations between cortical bone status at CT and the NCCN-IPI factors categorized age (ρ = 0.111, p = 0.261) categorized LDH ratio (ρ = −0.146, p = 0.138), presence of extranodal disease in major organs (φ = 0.146, p = 0.136), presence of Ann Arbor stage III/IV disease (φ = 0.048, p = 0.625), and ECOG performance status (φ = −0.029, p = 0.769).
Patient follow-up
The median follow-up time of surviving patients was 1,454 days (range, 134–3,597 days). Of 105 patients, 37 (35.2 %) experienced disease relapse/death, and 33 patients (31.4 %) died (Table 1).
Prognostic value of tumor-induced cortical bone destruction at CT
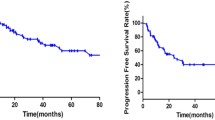
Patients with tumor-induced cortical bone destruction at CT had no significantly different PFS and OS than those without (log-rank test, p = 0.341 and p = 0.554, respectively) (Fig. 3). PFS and OS were significantly worse in patients with a positive BMB (log-rank test, p = 0.001 and p = 0.001) (Fig. 4) and in patients with a high-risk NCCN-IPI score (log-rank test, p < 0.001 and p < 0.001, respectively) (Fig. 5). Univariate Cox regression analysis indicated that cortical bone status at CT was no significant predictor of either PFS or OS (p = 0.358 and p = 0.560, respectively), whereas BMB findings (p = 0.002 and p = 0.013, respectively) and dichotomized NCCN-IPI risk strata (p = 0.002 and p = 0.003, respectively) were significant predictors of both PFS and OS (Table 2). In the multivariate Cox proportional hazards model, only the dichotomized NCCN-IPI score was an independent predictive factor of PFS and OS (p = 0.004 and p = 0.003, respectively) (Table 3).
Kaplan–Meier curves for PFS (a) and OS (b) of patients with tumor-induced cortical destruction at CT vs. patients without tumor-induced cortical destruction at CT. Patients with tumor-induced cortical destruction at CT had no significantly different PFS and OS than those without (log-rank test, p = 0.341 and p = 0.554, respectively)
Discussion
The results of this study show that the incidence of tumor-induced cortical bone destruction is low (i.e., less than 10 %) in newly diagnosed DLBCL. Interestingly, cortical bone destruction was observed in only 16 % (three out of 19 patients) with BMB-proven bone marrow involvement. Thus, in most patients, bone marrow involvement does not result in osteolysis. Most importantly, tumor-induced cortical bone destruction is not associated with any of the established NCCN-IPI factors, and is not associated with a worse PFS or OS. It can be speculated that although cortical bone destruction indicates an aggressive tumor growth, it does not indicate the presence of an R-CHOP-resistant phenotype. On the other hand, both BMB findings and the (dichotomized) NCCN-IPI risk strata were associated with PFS and OS, with BMB-positive patients and patients with high-risk NCCN-IPI scores showing worse outcome than BMB-negative patients and patients with low-risk NCCN-IPI scores, respectively. However, unlike BMB status, only the NCCN-IPI risk score remained as an independent predictive factor of both PFS and OS, which is in line with the recent publication by Zhou et al. [3]. The latter finding can be explained by the fact that BMB status is already part of the NCCN-IPI [3].
Given the fact that approximately one-third of patients with DLBCL still develop relapsed/refractory disease [1, 2], efforts are being made to identify new prognostic biomarkers that improve risk stratification in this disease. Unlike imaging-based bone marrow involvement, the presence of histologically confirmed bone marrow involvement is already an established negative prognostic factor in DLBCL, and is part of the current NCCN-IPI [3]. A disadvantage of BMB, however, is the fact that it assesses only a very small part of the bone marrow, thus being prone to sampling errors. Cross-sectional whole-body imaging modalities like FDG-PET and magnetic resonance imaging (MRI) have the advantage of being able to visualize the entire bone marrow, and it was hoped that bone marrow assessment with these techniques would improve prognostication. Although the role of MRI in this setting needs further investigation [17], the majority of studies on this topic have indicated that FDG-PET/CT-based bone assessment has no prognostic implications in DLBCL [6–8, 18]. CT is not an appropriate technique to evaluate the bone marrow [9], but is an ideal method to evaluate the bony cortex. Although lymphoma-induced cortical bone destruction indicates aggressive tumor growth, the hypothesis that this would be associated with a poorer outcome was not supported by the findings of the present study.
The prognostic impact of cortical bone destruction has been examined previously by Lee et al. [19]. Their study included patients diagnosed with DLBCL between 1994 and 2009. The PFS of 60 patients with bone lesions was compared with those of 181 patients with stage IV disease and similar IPI scores but without bone destruction. The PFS was not significantly different (p = 0.457) between two groups. Lee et al. [19] concluded that bone involvement may not have an additive adverse impact on the prognosis of Ann Arbor stage IV DLBCL, both in their entire study population (which included patients treated with different therapies) as well as in the subgroup of patients treated with R-CHOP. However, drawbacks of the study by Lee et al. [19] are that criteria for bone involvement were not reported, that the prognostic impact of bone involvement was assessed using matched controls rather than assessing the additional impact in the entire group of DLBCL patients over de NCCN-IPI and other risk factors using Cox regression, and that controls were selected on the basis of the old IPI [20] rather than the recently published NCCN-IPI [3].
This study had several limitations. First, the number of patients with cortical bone destruction was low. A future study with sufficient power may be necessary to confirm the present findings. Second, although the majority of patients had undergone full-dose CT, several patients received a low-dose CT scan only, as part of their FDG-PET/CT examination. In addition, a smooth reconstruction kernel was used and CT images were reconstructed to 5-mm slices. These factors may have had a negative impact on bony lesion detection. Third, although the NCCN-IPI defines four risk groups (low risk, low-intermediate risk, high-intermediate risk, and high risk), patients were dichotomized in low-risk (including low and low-intermediate risk patients) and high-risk (including high-intermediate and high risk patients) groups for survival analyses, because of the relatively limited sample size of the present study.
In conclusion, the presence of tumor-induced cortical bone destruction at CT was not found to have any no prognostic implications in newly diagnosed DLBCL. The NCCN-IPI remains the most important method for the prognostication of this disease.
References
Armitage JO. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin Proc. 2012;87(2):161–71.
Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program. 2011;2011:498–505.
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–42.
Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, Andre M, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii78–82.
Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2014;41(3):565–74.
Hong J, Lee Y, Park Y, Kim SG, Hwang KH, Park SH, et al. Role of FDG-PET/CT in detecting lymphomatous bone marrow involvement in patients with newly diagnosed diffuse large B-cell lymphoma. Ann Hematol. 2012;91(5):687–95.
Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122(1):61–7.
Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Bone marrow F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol. 2014.
Vinnicombe SJ, Reznek RH. Computerised tomography in the staging of Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2003;30 Suppl 1:S42–55.
Bohndorf K, Imhof H, Pope TL. Musculoskeletal imaging: a concise multimodality approach. New York: Thieme Medical Publishers; 2001.
Tatsumi M, Cohade C, Nakamoto Y, Fishman EK, Wahl RL. Direct comparison of FDG PET and CT findings in patients with lymphoma: initial experience. Radiology. 2005;237(3):1038–45.
de Jong PA, van Ufford HM, Baarslag HJ, de Haas MJ, Wittebol SH, Quekel LG, et al. CT and 18 F-FDG PET for noninvasive detection of splenic involvement in patients with malignant lymphoma. AJR Am J Roentgenol. 2009;192(3):745–53.
Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55(6):368–76.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86.
Privitera GJ. Student study guide with SPSS workbook for statistics for the behavioral sciences: SAGE publications, Inc., 2012.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Adams HJ, Kwee TC, Lokhorst HM, Westerweel PE, Fijnheer R, Kersten MJ, et al. Potential prognostic implications of whole-body bone marrow MRI in diffuse large B-cell lymphoma patients with a negative blind bone marrow biopsy. J Magn Reson Imaging. 2014;39(6):1394–400.
Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18 F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54(8):1244–50.
Lee HY, Kim SJ, Kim K, Ko YH, Kim WS. Bone involvement in patients with stage IV diffuse large B-cell lymphoma: does it have a prognostic value? Leuk Lymphoma. 2012;53(1):173–5.
A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329 (14):987–994.
Funding
This project was financially supported by an Alpe d’HuZes/Dutch Cancer Society Bas Mulder Award for T.C.K. (grant number 5409). Data collection, data analysis, and interpretation of data, writing of the paper, and decision to submit were left to the authors’ discretion and were not influenced by Alpe d’HuZes/Dutch Cancer Society.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Adams, H.J.A., de Klerk, J.M.H., Fijnheer, R. et al. Does the presence of tumor-induced cortical bone destruction at CT have any prognostic value in newly diagnosed diffuse large B-cell lymphoma?. Skeletal Radiol 44, 687–694 (2015). https://doi.org/10.1007/s00256-015-2102-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2102-z