Abstract
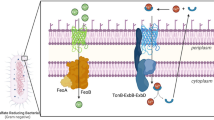
This study describes the thiosulfate-supported respiratory electron transport activity of Thiomonas bhubaneswarensis strain S10 (DSM 18181T). Whole-genome sequence analysis revealed the presence of complete sox (sulfur oxidation) gene cluster (soxCDYZAXB) including the sulfur oxygenase reductase (SOR), sulfide quinone reductase (SQR), sulfide dehydrogenase (flavocytochrome c (fcc)), thiosulfate dehydrogenase (Tsd), sulfite dehydrogenase (SorAB), and intracellular sulfur oxidation protein (DsrE/DsrF). In addition, genes encoding respiratory electron transport chain components viz. complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (ubiquinone-cytochrome c reductase), and various types of terminal oxidases (cytochrome c and quinol oxidase) were identified in the genome. Using site-specific electron donors and inhibitors and by analyzing the cytochrome spectra, we identified the shortest thiosulfate-dependent electron transport chain in T. bhubaneswarensis DSM 18181T. Our results showed that thiosulfate supports the electron transport activity in a bifurcated manner, donating electrons to quinol (bd) and cytochrome c (Caa 3 ) oxidase; these two sites (quinol oxidase and cytochrome c oxidase) also showed differences in their phosphate esterification potential (oxidative phosphorylation efficiency (P/O)). Further, it was evidenced that the substrate-level phosphorylation is the major contributor to the total energy budget in this bacterium.






Similar content being viewed by others
References
Assempour M, Hill BC (1997) Cyanide binding to different redox states of the cytochrome caa3 complex from Bacillus subtilis; a member of the cytochrome oxidase super-family of enzymes. Biochim Biophys Acta-Bioenergetics 1320:175–187
Beffa T, Berczy M, Aragno M (1992) Inhibition of respiratory oxidation of elemental sulfur (S0) and thiosulfate in Thiobacillus versutus and another sulfur-oxidizing bacterium. FEMS Microbiol Lett 90:123–127
Bennett S (2004) Solexa Ltd. Pharmacogenomics 5:433–438
Berglund F, Sorbo B (1960) Turbidimetric analysis of inorganic sulfate in serum, plasma and urine. Scand J Clin Lab Invest 12:147–153
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brasseur G, Levican G, Bonnefoy V, Holmes D, Jedlicki E, Lemesle-Meunier D (2004) Apparent redundancy of electron transfer pathways via bc(1) complexes and terminal oxidases in the extremophilic chemolithoautotrophic Acidithiobacillus ferrooxidans. Biochim Biophys Acta 1656:114–126
Dam B, Mandal S, Ghosh W, Das Gupta SK, Roy P (2007) The S4-intermediate pathway for the oxidation of thiosulfate by the chemolithoautotroph Tetrathiobacter kashmirensis and inhibition of tetrathionate oxidation by sulfite. Res Microbiol 158:330–338
Esposti MD (1989) Prediction and comparison of the haem-binding sites in membrane haemoproteins. Biochim Biophys Acta 977:249–265
Friedrich CG, Bardischewsky F, Rother D, Quentmeier A, Fischer J (2005) Prokaryotic sulfur oxidation. Curr Opin Microbiol 8:253–259
Gel’man NS, Lukoyanova MA, Ostrovskii DN (1967) The respiratory chain of bacteria. pp 71–159. In respiration and phosphorylation of bacteria. Springer. doi:10.1007/978-1-4899-5526-5
Ghosh W, Dam B (2009) Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol Rev 33:999–1043
Gleen H, Quastel JH (1953) Sulphur metabolism in soil. Appl Microbiol 1:70–77
Gnerre S, Maccallum I, Przybylski D, Ribeiro FJ, Burton JN, Walker BJ, Sharpe T, Hall G, Shea TP, Sykes S, Berlin AM, Aird D, Costello M, Daza R, Williams L, Nicol R, Gnirke A, Nusbaum C, Lander ES, Jaffe DB (2011) High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc Natl Acad Sci U S A 108:1513–1518
Grogan DW (1984) Interaction of respiration and luminescence in a common marine bacterium. Arch Microbiol 137:159–162
Guerrero MA, Makemson JC (1989) The cytochromes of luminous bacteria and their coupling to bioluminescence. Curr Microbiol 18:67–73
Hallberg KB, Dopson M, Lindstrom EB (1996) Reduced sulfur compound oxidation by Thiobacillus caldus. J Bacteriol 178:6–11
Hensen D, Sperling D, Truper HG, Brune DC, Dahl C (2006) Thiosulphate oxidation in the phototrophic sulphur bacterium Allochromatium vinosum. Mol Microbiol 62:794–810
Houghton JL, Foustoukos D, Flynn TM, Vetriani C, Bradley AS, Fike DA (2016) Thiosulfate oxidation by Thiomicrospira thermophila: metabolic flexibility in response to ambient geochemistry. Environ Microbiol. doi:10.1111/1462-2990.13232
Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119
Ikonomidis A, Tsakris A, Kanellopoulou M, Maniatis AN, Pournaras S (2008) Effect of the proton motive force inhibitor carbonyl cyanide-m-chlorophenylhydrazone (CCCP) on Pseudomonas aeruginosa biofilm development. Lett Appl Microbiol 47:298–302
Izawa S, Pan RL (1978) Photosystem I electron transport and phosphorylation supported by electron donation to the plastoquinone region. Biochem Biophys Res Commun 83:1171–1177
Junemann S (1997) Cytochrome bd terminal oxidase. Biochim Biophys Acta 1321:107–127
Kelly DP, Chambers LA, Trudinger PA (1969) Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal Chem 41:898–901
Kelly DP, Shergill JK, Lu WP, Wood AP (1997) Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Van Leeuwenhoek 71:95–107
Kikumoto M, Nogami S, Kanao T, Takada J, Kamimura K (2013) Tetrathionate-forming thiosulfate dehydrogenase from the acidophilic, chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. Appl Environ Microbiol 79:113–120
Loya S, Yankofsky SA, Epel BL (1982) Lithotrophy to organotrophy conversion in Thiobacillus A2. Microbiology 128:865–874
Lu W-P, Kelly DP (1983) Thiosulphate oxidation, electron transport and phosphorylation in cell-free systems from Thiobacillus A2. Microbiology 129:1661–1671
Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC (2009) IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 25:2271–2278
Masau RJ, Oh JK, Suzuki I (2001) Mechanism of oxidation of inorganic sulfur compounds by thiosulfate-grown Thiobacillus thiooxidans. Can J Microbiol 47:348–358
Meulenberg R, Pronk JT, Hazeu W, Bos P, Kuenen JG (1992) Oxidation of reduced sulphur compounds by intact cells of Thiobacillus acidophilus. Arch Microbiol 157:161–168
Meulenberg R, Scheer EJ, Pronk JT, Hazeu W, Bos P, Kuenen JG (1993) Metabolism of tetrathionate in Thiobacillus acidophilus. FEMS Microbiol Lett 112:167–172
Meyer B, Imhoff JF, Kuever J (2007) Molecular analysis of the distribution and phylogeny of the soxB gene among sulfur-oxidizing bacteria—evolution of the Sox sulfur oxidation enzyme system. Environ Microbiol 9:2957–2977
Muller FH, Bandeiras TM, Urich T, Teixeira M, Gomes CM, Kletzin A (2004) Coupling of the pathway of sulphur oxidation to dioxygen reduction: characterization of a novel membrane-bound thiosulphate:quinone oxidoreductase. Mol Microbiol 53:1147–1160
Panda SK, Jyoti V, Bhadra B, Nayak KC, Shivaji S, Rainey FA, Das SK (2009) Thiomonas bhubaneswarensis sp. nov., an obligately mixotrophic, moderately thermophilic, thiosulfate-oxidizing bacterium. Int J Syst Evol Microbiol 59:2171–2175
Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC (2010) GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 7:455–457
Pronk JT, Meulenberg R, Hazeu W, Bos P, Kuenen JG (1990) Oxidation of reduced inorganic sulphur compounds by acidophilic thiobacilli. FEMS Microbiol Lett 75:293–306
Richardson DJ (2000) Bacterial respiration: a flexible process for a changing environment. Microbiology 146:551–571
Sambrook J, Fritsch EF, Maniatis TA (1989) In molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Siletsky SA, Belevich I, Soulimane T, Verkhovsky MI, Wikstrom M (2013) The fifth electron in the fully reduced caa(3) from Thermus thermophilus is competent in proton pumping. Biochim Biophys Acta 1827:1–9
Sorokin DY, Cherepanov A, De Vries S, Kuenen GJ (1999) Identification of cytochrome c oxidase in the alkaliphilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium ‘Thioalcalomicrobium aerophilum’ strain AL 3. FEMS Microbiol Lett 179:91–99
Suzuki I (1999) Oxidation of inorganic sulfur compounds: chemical and enzymatic reactions. Can J Microbiol 45:97–105
Wentzien S, Sand W (1999) Polythionate metabolism in Thiomonas intermedia K12. Process Metallurgy 9:787–797
Yang NC, Ho WM, Chen YH, Hu ML (2002) A convenient one-step extraction of cellular ATP using boiling water for the luciferin-luciferase assay of ATP. Anal Biochem 306:323–327
Yin H, Zhang X, Li X, He Z, Liang Y, Guo X, Hu Q, Xiao Y, Cong J, Ma L, Niu J, Liu X (2014) Whole-genome sequencing reveals novel insights into sulfur oxidation in the extremophile Acidithiobacillus thiooxidans. BMC Microbiol 14:179
Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829
Acknowledgements
This work was supported in part by the funding received from the Department of Biotechnology, Government of India (D.O. no. BT/PR9712/NBD/52/91/2007), to SKD. Author KDN acknowledges the Council of Scientific and Industrial Research, Government of India, for providing the research fellowship. Thanks are due to Director, Institute of Life Sciences, for offering an adjunct faculty to SCS. The sequence data used in the study was produced by the US Department of Energy Joint Genome Institute (http://www.jgi.doe.gov/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 645 kb)
Rights and permissions
About this article
Cite this article
Narayan, K.D., Sabat, S.C. & Das, S.K. Mechanism of electron transport during thiosulfate oxidation in an obligately mixotrophic bacterium Thiomonas bhubaneswarensis strain S10 (DSM 18181T). Appl Microbiol Biotechnol 101, 1239–1252 (2017). https://doi.org/10.1007/s00253-016-7958-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7958-x