Abstract
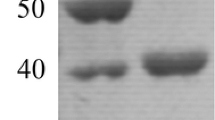
A novel endoglucanase encoding gene was cloned from Alicyclobacillus vulcanalis and expressed in E. coli. The deduced amino acid sequence showed highest identity with α-l-arabinofuranosidase-like proteins from glycoside hydrolase family 51. The recombinant enzyme was purified by affinity chromatography and characterised in terms of its potential suitability for lignocellulose hydrolysis at high temperature in the production of bioethanol. The purified enzyme displayed maximum activity at 80 °C and pH 3.6–4.5. Tween 20 was found to have a beneficial effect on enzyme activity and thermal stability. When incubated in the presence of 0.1 % Tween 20, the enzyme retained full activity after 72 h at 70 °C and 78 % of original activity after 72 h at 75 °C. Maximum activity was observed on carboxymethyl cellulose, and the purified enzyme also hydrolysed lichenan, barley β-glucan and xylan. The purified enzyme decreased the viscosity of carboxymethyl cellulose when assessed at 70–85 °C and was capable of releasing reducing sugars from acid-pretreated straw at 70 and 75 °C. The results indicate the potential suitability of the enzyme for industrial application in the production of cellulosic bioethanol.






Similar content being viewed by others
References
Bai YG, Wang JS, Zhang ZF, Shi PJ, Luo HY, Huang HQ, Luo CL, Yao B (2010a) Expression of an extremely acidic β-1,4-glucanase from thermoacidophilic Alicyclobacillus sp. A4 in Pichia pastoris is improved by truncating the gene sequence. Microb Cell Fact 93(3). doi: 10.1186/1475-2859-9-33
Bai Y, Wang J, Zhang Z, Shi P, Luo H, Huang H, Feng Y, Yao B (2010b) Extremely acidic beta-1,4-glucanase, CelA4, from thermoacidophilic Alicyclobacillus sp. A4 with high protease resistance and potential as a pig feed additive. J Agric Food Chem 58(3):1970–1975. doi:10.1021/jf9035595
Bai YG, Wang JS, Zhang ZF, Shi PJ, Luo HY, Huang HQ, Luo CL, Yao B (2010c) A novel family 9 beta-1,3(4)-glucanase from thermoacidophilic Alicyclobacillus sp. A4 with potential applications in the brewing industry. Appl Microbiol Biotechnol 87(1):251–259. doi:10.1007/s00253-010-2452-3
Bathgate G (1979) The institute of brewing analysis committee the determination of endo-β-glucanase activity in malt. J Inst Brew 85:92–94. doi:10.1002/j.2050-0416.1979.tb06833.x
Bhalla A, Bansal N, Kumar S, Bischoff KM, Sani RK (2013) Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour Technol 128:751–759. doi:10.1016/j.biortech.2012.10.145
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye-binding. Anal Biochem 72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3
Eckert K, Schneider E (2003) A thermoacidophilic endoglucanase (CelB) from Alicyclobacillus acidocaldarius displays high sequence similarity to arabinofuranosidases belonging to family 51 of glycoside hydrolases. Eur J Biochem 270(17):3593–3602. doi:10.1046/j.1432-1033.2003.03744.x
Eckert K, Zielinski F, Lo Leggio L, Schneider E (2002) Gene cloning, sequencing, and characterization of a family 9 endoglucanase (CelA) with an unusual pattern of activity from the thermoacidophile Alicyclobacillus acidocaldarius ATCC27009. Appl Microbiol Biotechnol 60(4):428–436. doi:10.1007/s00253-002-1131-4
Elleuche S, Schroder C, Sahm K, Antranikian G (2014) Extremozymes-biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol 29:116–123. doi:10.1016/j.copbio.2014.04.003
Eriksson T, Borjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 31(3):353–364. doi:10.1016/s0141-0229(02)00134-5
Kaar WE, Holtzapple MT (1998) Benefits from Tween during enzymic hydrolysis of corn stover. Biotechnol Bioeng 59(4):419–427. doi:10.1002/(SICI)1097-0290(19980820)59:4<419::AID-BIT4>3.3.CO;2-9
Karnaouri AC, Topakas E, Christakopoulos P (2014) Cloning, expression, and characterization of a thermostable GH7 endoglucanase from Myceliophthora thermophila capable of high-consistency enzymatic liquefaction. Appl Microbiol Biotechnol 98(1):231–242. doi:10.1007/s00253-013-4895-9
Kristensen JB, Borjesson J, Bruun MH, Tjerneld F, Jorgensen H (2007) Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb Technol 40(4):888–895. doi:10.1016/j.enzmictec.2006.07.014
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 227(5259):680–685. doi:10.1038/227680a0
Liu D, Zhang R, Yang X, Xu Y, Tang Z, Tian W, Shen Q (2011) Expression, purification and characterization of two thermostable endoglucanases cloned from a lignocellulosic decomposing fungi Aspergillus fumigatus Z5 isolated from compost. Protein Expression Purif 79(2):176–186. doi:10.1016/j.pep.2011.06.008
McCleary B (2001) Analysis of Feed Enzymes. In: Bedford M, Partridge G (eds) Enzymes in farm animal nutrition. CABI Publishing, Oxon, pp 85–107
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38(4):522–550. doi:10.1016/j.pecs.2012.02.002
Miller GL (1959) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. doi:10.1021/ac60147a030
Miller PS, Blum PH (2010) Extremophile-inspired strategies for enzymatic biomass saccharification. Environ Technol 31(8–9):1005–1015. doi:10.1080/09593330903536113
Modenbach AA, Nokes SE (2012) The use of high-solids loadings in biomass pretreatment—a review. Biotechnol Bioeng 109(6):1430–1442. doi:10.1002/bit.24464
Morana A, Esposito A, Maurelli L, Ruggiero G, Ionata E, Rossi M, La Cara F (2008) A novel thermoacidophilic cellulase from Alicyclobacillus acidocaldarius. Protein Pept Lett 15(9):1017–1021. doi:10.2174/092986608785849209
Pribowo A, Arantes V, Saddler JN (2012) The adsorption and enzyme activity profiles of specific Trichoderma reesei cellulase/xylanase components when hydrolyzing steam pretreated corn stover. Enzyme Microb Technol 50(3):195–203. doi:10.1016/j.enzmictec.2011.12.004
Rubingh DN (1996) The influence of surfactants on enzyme activity. Curr Opin Colloid Interface Sci 1(5):598–603
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, New York
Simbahan J, Drijber R, Blum P (2004) Alicyclobacillus vulcanalis sp nov., a thermophilic, acidophilic bacterium isolated from Coso Hot Springs, California, USA. Int J Syst Evol Microbiol 54:1703–1707. doi:10.1099/ijs. 0.03012-0
Singhania RR, Sukumaran RK, Patel AK, Larroche C, Pandey A (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol 46(7):541–549. doi:10.1016/j.enzmictec.2010.03.010
Skovgaard PA, Jorgensen H (2013) Influence of high temperature and ethanol on thermostable lignocellulolytic enzymes. J Ind Microbiol Biotechnol 40(5):447–456. doi:10.1007/s10295-013-1248-8
Szijarto N, Horan E, Zhang J, Puranen T, Siika-aho M, Viikari L (2011a) Thermostable endoglucanases in the liquefaction of hydrothermally pretreated wheat straw. Biotechnol Biofuels 4(2). doi: 10.1186/1754-6834-4-2
Szijarto N, Siika-aho M, Sontag-Strohm T, Viikari L (2011b) Liquefaction of hydrothermally pretreated wheat straw at high-solids content by purified Trichoderma enzymes. Bioresour Technol 102(2):1968–1974. doi:10.1016/j.biortech.2010.09.012
Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6. doi: 910.1186/1475-2859-6-9
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv 30(6):1458–1480. doi:10.1016/j.biotechadv.2012.03.002
Viikari L, Alapuranen M, Puranen T, Vehmaanpera J, Siika-Aho M (2007) Thermostable enzymes in lignocellulose hydrolysis. In: Olsson L (ed) Biofuels, Vol 108. Springer Berlin Heidelberg, pp 121–145
Viikari L, Vehmaanpera J, Koivula A (2012) Lignocellulosic ethanol: from science to industry. Biomass Bioenergy 46:13–24. doi:10.1016/j.biombioe.2012.05.008
Wang K, Luo H, Bai Y, Shi P, Huang H, Xue X, Yao B (2014) A thermophilic endo-1,4-beta-glucanase from Talaromyces emersonii CBS394.64 with broad substrate specificity and great application potentials. Appl Microbiol Biotechnol 98(16):7051–7060. doi:10.1007/s00253-014-5680-0
Yoon SH, Robyt JF (2005) Activation and stabilization of 10 starch-degrading enzymes by Triton X-100, polyethylene glycols, and polyvinyl alcohols. Enzyme Microb Technol 37(5):556–562. doi:10.1016/j.enzmictec.2005.04.002
Zhang F, Chen JJ, Ren WZ, Nie GX, Ming H, Tang SK, Li WJ (2011) Cloning, expression and characterization of an alkaline thermostable GH9 endoglucanase from Thermobifida halotolerans YIM 90462(T). Bioresour Technol 102(21):10143–10146. doi:10.1016/j.biortech.2011.08.019
Acknowledgments
This work was funded as part of the Science, Technology, Research and Innovation for the Environment (STRIVE) Programme 2007–2013. The programme is financed by the Irish Government under the National Development Plan 2007–2013, and it is administered on behalf of the DEHLG by the Environmental Protection Agency (EPA) which has the statutory function of coordinating and promoting environmental research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 36 kb)
Rights and permissions
About this article
Cite this article
Boyce, A., Walsh, G. Characterisation of a novel thermostable endoglucanase from Alicyclobacillus vulcanalis of potential application in bioethanol production. Appl Microbiol Biotechnol 99, 7515–7525 (2015). https://doi.org/10.1007/s00253-015-6474-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6474-8