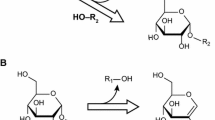
Abstract
Our investigation of the catalytic properties of Saccharomyces cerevisiae α-glucosidase (AGL) using hydroxybenzyl alcohol (HBA) isomers as transglucosylation substrates and their glucosides in hydrolytic reactions demonstrated interesting findings pertaining to the aglycon specificity of this important enzyme. AGL specificity increased from the para(p)- to the ortho(o)-HBA isomer in transglucosylation, whereas such AGL aglycon specificity was not seen in hydrolysis, thus indicating that the second step of the reaction (i.e., binding of the glucosyl acceptor) is rate-determining. To study the influence of substitution pattern on AGL kinetics, we compared AGL specificity, inferred from kinetic constants, for HBA isomers and other aglycon substrates. The demonstrated inhibitory effects of HBA isomers and their corresponding glucosides on AGL-catalyzed hydrolysis of p-nitrophenyl α-glucoside (PNPG) suggest that HBA glucosides act as competitive, whereas HBA isomers are noncompetitive, inhibitors. As such, we postulate that aromatic moieties cannot bind to an active site unless an enzyme-glucosyl complex has already formed, but they can interact with other regions of the enzyme molecule resulting in inhibition.






Similar content being viewed by others
References
Andreotti G, Giordano A, Tramice A, Mollo E, Trincone A (2006) Hydrolyses and transglycosylations performed by purified α-d-glucosidase of the marine mollusc Aplysia fasciata. J Biotechnol 122:274–284
Balba M, El-Hady N, Taha N, Rezki N, El Ashry ESH (2011) Inhibition of α-glucosidase and α-amylase by diaryl derivatives of imidazole-thione and 1,2,4-triazole-thiol. Eur J Med Chem 46:2596–2601
Chiba S, Shimomura T (1978) Diversity of substrate specificity of α-glucosidase. J Jap Soc Starch Sci 25:105–112
Copeland RA (2000) Enzymes: a practical introduction to structure, mechanism, and data analysis, 2nd edn. Wiley VCH, New York, p 126
Dhiman SB, Kamat JP, Naik DB (2009) Antioxidant activity and free radical scavenging reactions of hydroxybenzyl alcohols. Biochemical and pulse radiolysis studies. Chem Biol Interact 182:119–127
Dimitrijevic A, Velickovic D, Milosavic N, Bezbradica D (2012) Specificity of maltase to maltose in three different directions of reaction: hydrolytic, vanillyl alcohol glucoside and vanillyl alcohol isomaltoside synthesis. Biotechnol Prog 28:1450–1456
Du Z, Liu P, Shao W, Mao X, Ma L, Gu L, Huang Z, Chan ASC (2006) α-glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur J Med Chem 41:213–218
Eneyskaya EV, Golubev AM, Kachurin AM, Savel’ev AN, Neustroev KN (1997) Transglycosylation activity of a-d-galactosidase from Trichoderma reesei. An investigation of the active site. Carbohydr Res 305:83–91
Frandsen TP, Palcic MM, Svensson B (2002) Substrate recognition by three family 13 yeast α-glucosidases: evaluation of deoxygenated and conformationally biased isomaltosides. Eur J Biochem 269:728–734
Hakamata W, Muroi M, Kadokura K, Nishio T, Oku T, Kimura A, Chiba S, Takatsukia A (2005) Aglycon specificity profiling of a-glucosidases using synthetic probes. Bioorg Med Chem Lett 15:1489–1492
Halwachs W (1978) KM and Vmax from only one experiment. Biotechnol Bioeng 20:281–285
Howard S, Withers SG (1998) Labeling and identification of the postulated acid/base catalyst in the α-glucosidase from Saccharomyces cerevisiae using a novel bromoketone C-glycoside. Biogeosciences 37:3858–3864
Kren V, Martinkova L (2001) The role of glycosidic residues in biological activity. Curr Med Chem 8:1303–1328
Lee DS, Lee SH (2001) Genistein, a soy isoflavone, is a potent α-glucosidase inhibitor. FEBS Lett 501:84–86
Levvy GA, Marsh CA (1954) Competing substrates in enzyme research. Science 119:337–338
Lim EJ, Kang HJ, Jung HJ, Park EH (2007) Anti-angiogenic, anti-inflammatory and anti-nociceptive activity of 4-hydroxybenzyl alcohol. J Pharm Pharmacol 59:1235–1240
MacGregor EA, Janecek S, Svensson B (2001) Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim Biophys Acta 1546:1–20
McCarter JD, Withers SG (1996) Unequivocal identification of Asp-214 as the catalytic nucleophile of Saccharomyces cerevisiae alpha-glucosidase using 5-fluoro glycosyl fluorides. J Biol Chem 271:6889–6894
Milosavic N, Prodanovic R, Jankov R (2008) Stereoselectivity of α-glucosidase from baker’s yeast for transglucosylation reaction. J Biotechnol 136:S361–S362
Nakai H, Okuyama M, Kim YM, Saburi W, Wongchawalit J, Mori H, Chiba S, Kimura A (2005) Molecular analysis of α-glucosidase belonging to GH-family 31. Biologia 16:131–135
Nishizawa K, Amano Y, Isobe T, Nozaki K, Shiroishi M, Kanda T (2011) Aglycone specificity in transglycosylation of a xylanase produced from basidiomycete, Hypsizigus marmoreus during the mushroom cultivation. J Appl Glycosci 49:137–143
Ojha S, Mishra S, Kapoor S, Chand S (2013) Synthesis of hexyl α-glucoside and α-polyglucosides by a novel Microbacterium isolate. Appl Microbiol Biotechnol 97:5293–5301
Okamura K, Sakamoto M, Ishikura T (1980) PS-5 inhibition of a beta-lactamase from Proteus vulgaris. J Antibiot 33(3):293–302
Pavlović M, Dimitrijević A, Trbojević J, Milosavić N, Gavrović- Jankulović M, Bezbradica D, Veličković D (2013) Study of transglucosylation kinetics in enzymatic synthesis of benzyl alcohol glucoside by α-glucosidase from S.cerevisiae. Russ J Phys Chem A 87:2285–2288
Prodanovic RM, Milosavic N, Sladic D, Zlatovic M, Bozic B, Velickovic T (2005) Transglucosylation of hydroquinone catalysed by alpha-glucosidase from baker’s yeast. J Mol Catal B Enzym 35:142–146
Prodanovic R, Milosavic N, Jovanovic S, Cirkovic-Velickovic T, Vujcic Z, Jankov RM (2006a) Stabilization of α-glucosidase in organic solvents by immobilization on macroporous poly(GMA-co-EGDMA) with different surface characteristics. J Serb Chem Soc 71:339–347
Prodanovic R, Milosavic N, Jovanovic S, Prodanovic O, Cirkovic-Velickovic T, Vujcic Z, Jankov RM (2006b) Activity and stability of soluble and immobilized α-glucosidase from baker’s yeast in cosolvent systems. Biocatal Biotransform 24:195–200
Sato T, Nakagawa H, Kurosu J, Yoshida K, Tsugane T, Susumu S, Kirimura K, Kino K, Usami S (2000) α-Anomer-selective glucosylation of (+)-catechin by the crude enzyme, showing glucosyl transfer activity, of Xanthomona scampestris VW-9701. J Biosci Bioeng 90:625–630
Shai LJ, Masoko P, Mokgotho MP, Magano SR, Mogale AM, Boaduo N, Eloff JN (2010) Yeast α glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S Afr J Bot 76:465–470
Shin HK, Kong JY, Lee JD, Lee TH (2000) Syntheses of hydroxybenzyl-α-glucosides by amyloglucosidase-catalyzed transglycosylation. Biotechnol Lett 22:321–325
Velickovic D, Dimitrijevic A, Bihelovic F, Bezbradica D, Jankov R, Milosavic N (2011a) A highly efficient diastereoselective synthesis of α-isosalicin by maltase from Saccharomyces cerevisiae. Process Biochem 46:1698–1702
Velickovic D, Dimitrijevic A, Bihelovic F, Jankov R, Milosavic N (2011b) Study of the kinetic parameters for synthesis and hydrolysis of pharmacologically active salicin isomer catalyzed by baker’s yeast maltase. Russ J Phys Chem A 85:2317–2321
Velickovic D, Dimitrijevic A, Bihelovic F, Bezbradica D, Knezevic-Jugovic Z, Milosavic N (2012) Novel glycoside of vanillyl alcohol, 4-hydroxy-3-methoxybenzyl-alpha-d-glucopyranoside: study of enzymatic synthesis, in vitro digestion and antioxidant activity. Bioprocess Biosyst Eng 35:1107–1115
Voragen GJA (1998) Technological aspects of food-related carbohydrates. Trends Food Sci Technol 9:328–335
Wehmeier UF, Piepersberg W (2004) Biotechnology and molecular biology of the α-glucosidase inhibitor acarbose. Appl Microbiol Biotechnol 63:613–625
Yao X, Mauldin R, Byers L (2003) Multiple sugar binding sites in α-glucosidase. Biochim Biophys Acta 1645:22–29
Acknowledgments
The authors are grateful for the financial support of the Ministry of Science of the Republic of Serbia (project nos. 172049, 046010, and 451-03-00605/2012-16/51) and FP7 Reg Pot FCUB ERA, GA No. 256716.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dušan, V., Nenad, M., Dejan, B. et al. The specificity of α-glucosidase from Saccharomyces cerevisiae differs depending on the type of reaction: hydrolysis versus transglucosylation. Appl Microbiol Biotechnol 98, 6317–6328 (2014). https://doi.org/10.1007/s00253-014-5587-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5587-9