Abstract
Background
Gadolinium-based contrast agents (GBCAs) have been used for magnetic resonance (MR) imaging over the last three decades. Recent reports demonstrated gadolinium retention in patients’ brains following intravenous administration. Since gadolinium is a highly toxic heavy metal, there is a potential for adverse effects from prolonged retention or deposition, particularly in children. For this reason, the Society (SPR) for Pediatric Radiology Quality and Safety committee conducted a survey to evaluate the current status of GBCAs usage among pediatric radiologists.
Objective
To assess the usage of GBCAs among SPR members.
Materials and methods
An online 15-question survey was distributed to SPR members. Survey questions pertained to the type of GBCAs used, protocoling workflow, requirement of renal function or pregnancy tests, and various clinical indications for contrast-enhanced MRI examinations.
Results
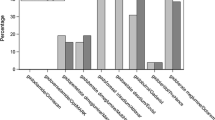
A total of 163 survey responses were compiled (11.1% of survey invitations), the majority of these from academic institutions in the United States. Ninety-four percent reported that MR studies are always or usually protocoled by pediatric radiologists. The most common GBCA utilized by survey respondents were Eovist (60.7%), Ablavar (45.4%), Gadovist (38.7%), Magnevist (34.4%) and Dotarem (32.5%). For several clinical indications, survey responses regarding GBCA administration were concordant with American College of Radiology (ACR) Appropriateness Criteria, including seizures, headache and osteomyelitis. For other indications, including growth hormone deficiency and suspected vascular ring, survey responses revealed potential overutilization of GBCAs when compared to ACR recommendations.
Conclusion
Survey results demonstrate that GBCAs are administered judiciously in children, yet there is an opportunity to improve their utilization with the goal of reducing potential future adverse effects.
Similar content being viewed by others
References
Port M, Idée JM, Medina C et al (2008) Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 21:469–490
Ramalho J, Semelka RC, Ramalho M et al (2015) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37:1192–1198
Kanal E, Maravilla K, Rowley HA (2014) Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 35:2215–2226
Khawaja AZ, Cassidy DB, Al Shakarchi J et al (2015) Revisiting the risks of MRI with gadolinium based contrast agents-review of literature and guidelines. Insights Imaging 6:553–558
ACR manual on contrast media version 10.1 (2015) http://www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed 7 Dec 2016
Gibby WA, Gibby KA, Gibby WA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol 39:138–142
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol 41:272–278
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Matsuda M, Oba H et al (2015) Gadolinium deposition after contrast-enhanced MR Imaging. Radiology 277:924–925
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Leyendecker JR (2009) Gadoxetate disodium for contrast magnetic resonance imaging of the liver. Gastroenterol Hepatol 5:698
Rigsby CK, Popescu AR, Nelson P et al (2015) Safety of blood pool contrast agent administration in children and young adults. AJR Am J Roentgenol 205:1114–1120
Trout AT, Dillman JR, Ellis JH et al (2011) Patterns of intravenous contrast material use and corticosteroid premedication in children--a survey of Society of Chairs of Radiology in Children’s Hospitals (SCORCH) member institutions. Pediatr Radiol 41:1272–1283
Hayes L, Coley B, Karmazyn B et al (2012) Headache – Child. ACR appropriateness criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Beaman F, von Herrmann P, Kransdorf M et al (2016); Suspected osteomyelitis, septic arthritis, or soft tissue infection. ACR Appropriateness Criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Seidenwurm D, Wippold F, Cornelius R et al (2012) Neuroendocrine Imaging. ACR Appropriateness Criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Ho V, Biko D, White R et al. (2011) Known or suspected congenital heart disease in the adult. ACR Appropriateness Criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 165 kb)
Rights and permissions
About this article
Cite this article
Blumfield, E., Moore, M.M., Drake, M.K. et al. Survey of gadolinium-based contrast agent utilization among the members of the Society for Pediatric Radiology: a Quality and Safety Committee report. Pediatr Radiol 47, 665–673 (2017). https://doi.org/10.1007/s00247-017-3807-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3807-z