Abstract
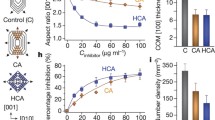
Crystal growth rates have been extensively studied in calcium oxalate monohydrate (COM) crystallization, because COM crystals are the principal component in most kidney stones. Constant composition methods are useful for studying growth rates, but fail to differentiate concurrent nucleation and aggregation events. A constant composition method coupled with particle size determinations that addresses this deficiency was previously published for a calcium phosphate system, and this method was extended to COM crystallization in this report. A seeded constant composition experiment was combined with particle size determination and a separate near-equilibrium aggregation experiment to separate effects of growth rate, nucleation, and aggregation in COM crystal formation and to test the effects of various inhibitors relevant to stone formation. With no inhibitors present, apparent COM growth rates were heavily influenced by secondary nucleation at low seed crystal additions, but growth-related aggregation increased at higher seed crystal densities. Among small molecule inhibitors, citrate demonstrated growth rate inhibition but enhanced growth-related aggregation, while magnesium did not affect COM crystallization. Polyanions (polyaspartate, polyglutamate, or osteopontin) showed strong growth rate inhibition, but large differences in nucleation and aggregation were observed. Polycations (polyarginine) did not affect COM crystal growth or aggregation. Mixtures of polyanions and polycations produced a complicated set of growth rate, nucleation, and aggregation behaviors. These experiments demonstrated the power of combining particle size determinations with constant composition experiments to fully characterize COM crystallization and to obtain detailed knowledge of inhibitor properties that will be critical to understanding kidney stone formation.





Similar content being viewed by others
References
Worcester EM, Beshensky AM, Hung L (1993) Nephrocalcin (NC) levels and calcium oxalate (CaOx) crystal growth inhibition in the urine of hypercalciuric and normocalciuric calcium stone formers (SF). J Amer Soc Neph 4:716
Ryall RL, Hibberd CM, Mazzachi BC, Marshall VR (1986) Inhibitory activity of whole urine: a comparison of urines from stone formers and healthy subjects. Clin Chim Acta 154:59–67
Romberg RW, Werness PG, Riggs BL, Mann KG (1986) Inhibition of hydroxyapatite crystal growth by bone-specific and other calcium-binding proteins. Biochemistry (NY) 25:1176–1180
Donnelly R, Boskey A (1989) The effect of gallium on seeded hydroxyapatite growth. Calcif Tissue Int 44:138–142
Dominguez-Vera JM, Gautron J, Garcia-Ruiz JM, Nys Y (2000) The effect of avian uterine fluid on the growth behavior of calcite crystals. Poult Sci 79:901–907
Falini G, Albeck S, Weiner S, Addadi L (1996) Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271:67–69
Kavanagh JP (2006) In vitro calcium oxalate crystallisation methods. Urol Res 34:139–145
Saw NK, Rao PN, Kavanagh JP (2008) A nidus, crystalluria and aggregation: key ingredients for stone enlargement. Urol Res 36:11–15
Tomson MB, Nancollas GH (1978) Mineralization kinetics: a constant composition approach. Science 200:1059–1060
Amjad Z, Koutsoukos P, Tomson MB, Nancollas GH (1978) The growth of hydroxyapatite from solution. A new constant composition method. J Dent Res 57:909
Beshensky AM, Wesson JA, Worcester EM, Sorokina EJ, Snyder CJ, Kleinman JG (2001) Effects of urinary macromolecules on hydroxyapatite crystal formation. J Am Soc Nephrol 12:2108–2116
Pampena DA, Robertson KA, Litvinova O, Lajoie G, Goldberg HA, Hunter GK (2004) Inhibition of hydroxyapatite formation by osteopontin phosphopeptides. Biochem J 378:1083–1087
Wang L, De Yoreo JJ, Guan X, Qiu SR, Hoyer JR, Nancollas GH (2006) constant composition studies verify the utility of the Cabrera-Vermilyea (C-V) model in explaining mechanisms of calcium oxalate monohydrate crystallization. Cryst Growth Des 6:1769–1775
Salimi MH, Heughebaert JC, Nancollas GH (1985) Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1:119–122
Koutsoukos P, Amjad Z, Tomson MB, Nancollas GH (1980) Crystallization of calcium phosphates. A constant composition study. J Am Chem Soc 102:1553–1557
Heughebaert JC, Nancollas GH (1984) Kinetics of crystallization of octacalcium phosphate. J Phys Chem 88:2478–2481
Amjad Z (1991) Inhibition of calcium fluoride crystal growth by polyelectrolytes. Langmuir 7:2405–2408
Kazmierczak TF, Tomson MB, Nancollas GH (1982) Crystal growth of calcium carbonate. A controlled composition kinetic study. J Phys Chem 86:103–107
Gebrehiwet TA, Redden GD, Fujita Y, Beig MS, Smith RW (2012) The Effect of the CO32− to Ca2+ Ion activity ratio on calcite precipitation kinetics and Sr2+ partitioning. Geochem Trans 13:1–11
Manoli F, Dalas E (2002) The crystallization of calcium carbonate on sodium cholate. J Mater Sci Mater Med 13:69–73
Lanzalaco AC, Sheehan ME, White DJ, Nancollas GH (1982) The mineralization inhibitory potential of urines: a constant composition approach. J Urol 128:845–849
Lanzalaco AC, Singh RP, Smesko SA, Nancollas GH, Sufrin G, Binette M, Binette JP (1988) The influence of urinary macromolecules on calcium oxalate monohydrate crystal growth. J Urol 139:190–195
White DJ, Coyle-Rees M, Nancollas GH (1988) Kinetic factors influencing the dissolution behavior of calcium oxalate renal stones: a constant composition study. Calcif Tissue Int 43:319–327
Sheehan ME, Nancollas GH (1980) Calcium oxalate crystal growth. A new constant composition method for modelling urinary stone formation. Invest Urol 17:446–450
Nancollas GH, Singh RP (1987) In vitro system for calcium stone formation: the constant composition model. Contrib Nephrol 58:49–58
Blomen LJMJ, Will EJ, Bijvoet OLM, van der Linden H (1983) Growth kinetics of calcium oxalate monohydrate: II. The variation of seed concentration. J Cryst Growth 64:306–315
Wesson JA, Ganne V, Beshensky AM, Kleinman JG (2005) Regulation by macromolecules of calcium oxalate crystal aggregation in stone formers. Urol Res 33:206–212
Howard JE, Thomas WC Jr, Barker LM, Smith LH, Wadkins CL (1967) The recognition and isolation from urine and serum of a peptide inhibitor to calcification. Johns Hopkins Med J 120:119–136
Dent CE, Sutor DJ (1971) Presence or absence of inhibitor of calcium-oxalate crystal growth in urine of normals and of stone formers. Lancet 775–778
Robertson WG, Peacock M, Nordin BEC (1973) Inhibitors of the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta 43:31–37
Worcester EM, Blumenthal SS, Beshensky AM, Lewand DL (1992) The calcium oxalate crystal growth inhibitor protein produced by mouse kidney cortical cells in culture is osteopontin. J Bone Miner Res 7:1029–1036
Manning G (1969) Limiting laws and counterion condensation in polyelectrolyte solutions I colligative properties. J Chem Phys 51:924
Veis A (1970) Biological polyelectrolytes. Biological Macromolecules Series. vol 3. Marcel Dekker, NY
Lemann J Jr, Pleuss JA, Worcester EM, Hornick L, Schrab D, Hoffmann RG (1996) Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int 49:200–208
Cao LC, Deng G, Boeve ER, de Bruijn WC, de Water R, Verkoelen CF, Romijn JC, Schroder FH (1996) Zeta potential measurement and particle size analysis for a better understanding of urinary inhibitors of calcium oxalate crystallization. Scan Microsc 10:401–411
Boeve ER, Cao LC, Deng G, de Bruijn WC, Schroder FH (1996) Effect of two new polysaccharides on growth, agglomeration and zeta potential of calcium phosphate crystals. J Urol 155:368–373
Jung T, Sheng X, Choi CK, Kim WS, Wesson JA, Ward MD (2004) Probing calcium oxalate monohydrate crystal growth and the role of macromolecular additives with in situ atomic force microscopy. Langmuir 20:8587–8596
Wesson JA, Worcester EM, Kleinman JG (2000) Role of anionic proteins in kidney stone formation: interaction between model anionic polypeptides and calcium oxalate crystals. J Urol 163:1343–1348
Guo S, Ward MD, Wesson JA (2002) Direct visualization of calcium oxalate monohydrate crystallization and dissolution with atomic force microscopy and the role of polymeric additives. Langmuir 18:4284–4291
Qiu SR, Wierzbicki A, Orme CA, Cody AM, Hoyer JR, Nancollas GH, Zepeda S, De Yoreo JJ (2004) Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc Natl Acad Sci USA 101:1811–1815
Chien YC, Masica DL, Gray JJ, Nguyen S, Vali H, McKee MD (2009) Modulation of calcium oxalate dihydrate growth by selective crystal-face binding of phosphorylated osteopontin and polyaspartate peptide showing occlusion by sectoral (compositional) zoning. J Biol Chem 284:23491–23501
Sheng X, Jung T, Wesson JA, Ward MD (2005) Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc Natl Acad Sci USA 102:267–272
Viswanathan P, Rimer JD, Kolbach AM, Ward MD, Kleinman JG, Wesson JA (2011) Calcium oxalate monohydrate aggregation induced by aggregation of desialylated Tamm-Horsfall protein. Urol Res. doi:10.1007/s00240-010-0353-7
Bitton R, Schmidt J, Biesalski M, Tu R, Tirrell M, Bianco-Peled H (2005) Self-assembly of model DNA-binding peptide amphiphiles. Langmuir
Priftis D, Laugel N, Tirrell M (2012) Thermodynamic characterization of polypeptide complex coacervation. Langmuir. doi:10.1021/la302729r
Umekawa T, Iguchi M, Kurita T (2001) The effect of osteopontin immobilized collagen granules in the seed crystal method. Urol Res
Scurr DS, Robertson WG (1986) Modifiers of calcium oxalate crystallization found in urine. III. Studies on the role of Tamm-Horsfall mucoprotein and of ionic strength. J Urol 136:505–507
Acknowledgments
We gratefully acknowledge the primary financial support provided in part by Merit Review and Career Development Awards from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Program (9305) and by The Medical College of Wisconsin (JAW). Additional financial support was provided in part by the National Institutes of Health/National Institute for Diabetes, Digestive, and Kidney Diseases (DK 48504) (JGK). We also gratefully acknowledge the technical support from Dr. Neil Mandel and coworkers at the VA National Crystal Identification Center, Milwaukee, WI, where crystal surface area measurements were performed by Dr. John Weissner and x-ray crystallography was performed by Ms. Kathy Fryjoff.
Conflict of interest
The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolbach-Mandel, A.M., Kleinman, J.G. & Wesson, J.A. Exploring calcium oxalate crystallization: a constant composition approach. Urolithiasis 43, 397–409 (2015). https://doi.org/10.1007/s00240-015-0781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0781-5