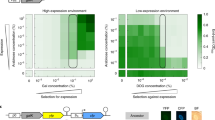
Abstract
We devised a molecular evolution procedure to evolve E. coli promoter sequences and applied it to observe an arbitrary, nonfunctional sequence evolving into functional promoters. In the experiments, DNA sequence variations were generated with error-prone PCR and were inserted in the promoter region of the cat (chloramphenicol acetyl transferase) gene on a plasmid. Upon transforming the cells, functional promoters on the plasmid were selected according to the chloramphenicol resistance. Within a few cycles of mutation-selection, promoters emerged, and the sequences converged into a small number of groups. In the process, the extended minus 10 type of promoters emerged quickly, and small deletions were often involved in adjusting the length between the −35 and the −10 elements. Our results also suggest a possible selection for promoter stability against mutation.












Similar content being viewed by others
References
Barne KA, Bown JA, Busby SJ, Minchin SD (1997) Region 2.5 of the Escherichia coli RNA polymerase sigma70 subunit is responsible for the recognition of the ‘extended−10’ motif at promoters. EMBO J 16:4034–4040
Berninger MS (1993) Use of exo-sample nucleotides in gene cloning. United States Patent and Trademark Office, Life Technologies, Inc
Burr T, Mitchell J, Kolb A, Minchin S, Busby S (2000) DNA sequence elements located immediately upstream of the −10 hexamer in Escherichia coli promoters: a systematic study. Nucleic Acids Res 28:1864–1870
Cadwell RC, Joyce GF (1992) Randomization of genes by PCR mutagenesis. PCR Methods Appl 2:28–33
Estrem ST, Gaal T, Ross W, Gourse RL (1998) Identification of an UP element consensus sequence for bacterial promoters. Proc Natl Acad Sci USA 95:9761–9766
Gaal T, Ross W, Estrem ST, Nguyen LH, Burgess RR, Gourse RL (2001) Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol Microbiol 42:939–954
Harley CB, Reynolds RP (1987) Analysis of E. coli promoter sequences. Nucleic Acids Res 15:2343–2361
Hawley DK, McClure WR (1983) Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res 11:2237–2255
Horwitz MS, Loeb LA (1986) Promoters selected from random DNA sequences. Proc Natl Acad Sci USA 83:7405–7409
Horwitz MS, Loeb LA (1988) DNA sequences of random origin as probes of Escherichia coli promoter architecture. J Biol Chem 263:14724–14731
Kumar A, Malloch RA, Fujita N, Smillie DA, Ishihama A, Hayward RS (1993) The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol 232:406–418
Lederberg J, Lederberg EM (1952) Replica plating and indirect selection of bacterial mutants. J. Bacteriol 63:399–406
Liu M, Tolstorukov M, Zhurkin V, Garges S, Adhya S (2004) A mutant spacer sequence between −35 and −10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc Natl Acad Sci USA 101:6911–6916
Oliphant AR, Struhl K (1987) The use of random-sequence oligonucleotides for determining consensus sequences. Methods Enzymol 155:568–582
Oliphant AR, Struhl K (1988) Defining the consensus sequences of E.coli promoter elements by random selection. Nucleic Acids Res 16:7673–7683
Pribnow D (1975) Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci USA 72:784–788
Raibaud O, Schwartz M (1984) Positive control of transcription initiation in bacteria. Annu Rev Genet 18:173–206
Rashtchian A, Berninger MS (1992) Use of exo-sample nucleotides in gene cloning. United States Patent and Trademark Office, Life Technologies, Inc
Rosenberg M, Court D (1979) Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet 13:319–353
Seeburg PH, Nusslein C, Schaller H (1977) Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem 74:107–113
Xu J, McCabe BC, Koudelka GB (2001) Function-based selection and characterization of base pair polymorphisms in a promoter of Escherichia coli RNA polymerase-sigma(70). J Bacteriol 183:2866–2873
Zaccolo M, Gherardi E (1999) The effect of high-frequency random mutagenesis on in vitro protein evolution: a study on TEM-1 beta-lactamase. J Mol Biol 285:775–783
Zaccolo M, Williams DM, Brown DM, Gherardi E (1996) An approach to random mutagenesis of DNA using mixtures of triphosphate derivatives of nucleoside analogues. J Mol Biol 255:589–603
Acknowledgments
We thank Christina Wakamoto (University of California, San Diego) for invaluable help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Reviewing Editior: Dr. Laura Landweber]
Rights and permissions
About this article
Cite this article
Liu, S., Libchaber, A. Some Aspects of E. coli Promoter Evolution Observed in a Molecular Evolution Experiment. J Mol Evol 62, 536–550 (2006). https://doi.org/10.1007/s00239-005-0128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-005-0128-x