Abstract
Introduction
We aimed to describe the clinico-radiological findings of patients with disorders of diencephalic–mesencephalic junction (DMJ) formation and midbrain anteroposterior patterning.
Methods
We reviewed the DMJ anatomy of 445 patients with brain malformations. Associated supra/infratentorial abnormalities and clinical findings were noted. Craniocaudal and anteroposterior diameters of midbrain, pons, medulla, vermis, and transverse cerebellar diameter were compared with age-matched controls. Post hoc tests were corrected according to Bonferroni (p B).
Results
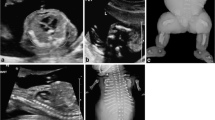
Two patterns of DMJ anomaly were identified in 12 patients (7 females, mean age 41 months). Type A was characterized by hypothalamic–mesencephalic fusion on axial plane, with possible midbrain ventral cleft (7 patients). Anteroposterior (p B = .006) and craniocaudal (p B = .027) diameters of the pons, craniocaudal diameter of the vermis (p B = .015), and transverse cerebellar diameter (p B = .011) were smaller than the controls. Corticospinal tract, basal ganglia, and commissural anomalies were also associated. Clinical findings included spastic-dystonic tetraparesis, hypothalamic dysfunction, epilepsy, and severe developmental delay. Type B was characterized by incomplete thalamic–mesencephalic cleavage on sagittal plane, with parenchymal bands connecting the interthalamic adhesion with the midbrain (five patients). Anteroposterior diameters of midbrain (p B = .002), pons (p B = .0004), and medulla (p B = .002) as well as the vermian anteroposterior (p B = .040) and craniocaudal diameters (p B = .014) were smaller than the controls. These patients were less neurologically impaired, most presenting mild developmental delay.
Conclusions
The spectrum of DMJ patterning defects is wide and may be associated with several brain malformations. Infratentorial brain structures should be carefully evaluated to better define the type of associated midbrain–hindbrain anomalies.





Similar content being viewed by others
References
Barkovich AJ, Millen KJ, Dobyns WB (2007) A developmental classification of malformations of the brainstem. Ann Neurol 62:625–639
Barkovich AJ, Millen KJ, Dobyns WB (2009) A developmental and genetic classification for midbrain-hindbrain malformations. Brain 132(Pt 12):3199–3230
Doherty D, Millen KJ, Barkovich AJ (2013) Midbrain and hindbrain malformations: advances in clinical diagnosis, imaging, and genetics. Lancet Neurol 12(4):381–393
Jissendi-Tchofo P, Severino M, Nguema-Edzang B, Toure C, Soto Ares G, Barkovich AJ (2015) Update on neuroimaging phenotypes of mid-hindbrain malformations. Neuroradiology 57(2):113–138
Bosemani T, Orman G, Boltshauser E, Tekes A, Huisman TA, Poretti A (2015) Congenital abnormalities of the posterior fossa. Radiographics 35(1):200–220
Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, Graham JM Jr, Maria BL, Barkovich AJ, Dobyns WB (2004) Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A 125A(2):125–134, discussion 117
Poretti A, Huisman TA, Scheer I, Boltshauser E (2011) Joubert syndrome and related disorders: spectrum of neuroimaging findings in 75 patients. AJNR Am J Neuroradiol 32(8):1459–1463
Rossi A, CatalaM BR, Di Comite R, Tortori-Donati P (2004) MR imaging of brain-stem hypoplasia in horizontal gaze palsy with progressive scoliosis. AJNR Am J Neuroradiol 25(6):1046–1048
Barth PG, Majoie CB, Caan MW, Weterman MA, Kyllerman M, Smit LM, Kaplan RA, Haas RH, Baas F, Cobben JM, Poll-The BT (2007) Pontine tegmental cap dysplasia: a novel brain malformation with a defect in axonal guidance. Brain 130(Pt 9):2258–2266
Jissendi-Tchofo P, Doherty D, McGillivray G, Hevner R, Shaw D, Ishak G, Leventer R, Barkovich AJ (2009) Pontine tegmental cap dysplasia: MR imaging and diffusion tensor imaging features of impaired axonal navigation. AJNR Am J Neuroradiol 30(1):113–119
Ishak GE, Dempsey JC, Shaw DW, Tully H, Adam MP, Sanchez-Lara PA, Glass I, Rue TC, Millen KJ, Dobyns WB, Doherty D (2012) Rhombencephalosynapsis: a hindbrain malformation associated with incomplete separation of midbrain and forebrain, hydrocephalus and a broad spectrum of severity. Brain 135(Pt 5):1370–1386
PCH Consortium, Namavar Y, Barth PG, Kasher PR, van Ruissen F, Brockmann K, Bernert G, Writzl K, Ventura K, Cheng EY, Ferriero DM, Basel-Vanagaite L, Eggens VR, Krägeloh-Mann I, De Meirleir L, King M, Graham JM Jr, von Moers A, Knoers N, Sztriha L, Korinthenberg R, Dobyns WB, Baas F, Poll-The BT (2011) Clinical, neuroradiological and genetic findings in pontocerebellar hypoplasia. Brain 134(Pt 1):143–156
Zaki MS, Saleem SN, Dobyns WB, Barkovich AJ, Bartsch H, Dale AM, Ashtari M, Akizu N, Gleeson JG, Grijalvo-Perez AM (2012) Diencephalic-mesencephalic junction dysplasia: a novel recessive brain malformation. Brain 135(Pt 8):2416–2427
Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB (2012) A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135(Pt 5):1348–1369
Severino M, Allegri AE, Pistorio A, Roviglione B, Di Iorgi N, Maghnie M, Rossi A (2014) Midbrain-hindbrain involvement in septo-optic dysplasia. AJNR Am J Neuroradiol 35(8):1586–1592
Martinez S, Wassef M, Alvarado-Mallart RM (1991) Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene en. Neuron 6(6):971–981
Nakamura H, Watanabe Y (2005) Isthmus organizer and regionalization of the mesencephalon and metencephalon. Int J Dev Biol 49(2–3):231–235
Scholpp S, Lohs C, Brand M (2003) Engrailed and Fgf8 act synergistically to maintain the boundary between diencephalon and mesencephalon. Development 130:4881–4893
McGinty D, Gong H, Suntsova N, Alam MN, Methippara M, Guzman-Marin R, Szymusiak R (2004) Sleep-promoting functions of the hypothalamic median preoptic nucleus: inhibition of arousal systems. Arch Ital Biol 142(4):501–509
Bahi-Buisson N, Poirier K, Fourniol F, Saillour Y, Valence S, Lebrun N, Hully M, Bianco CF, Boddaert N, Elie C, Lascelles K, Souville I, Bahi-Buisson N, Poirier K, Fourniol F, Saillour Y, Valence S, Lebrun N, Hully M, Bianco CF, Boddaert N, Elie C, Lascelles K, Souville I, LIS-Tubulinopathies Consortium, Beldjord C, Chelly J (2014) The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain 137(Pt 6):1676–1700
Kato M, Das S, Petras K, Kitamura K, Morohashi K, Abuelo DN, Barr M, Bonneau D, Brady AF, Carpenter NJ, Cipero KL, Frisone F, Fukuda T, Guerrini R, Iida E, Itoh M, Lewanda AF, Nanba Y, Oka A, Proud VK, Saugier-Veber P, Schelley SL, Selicorni A, Shaner R, Silengo M, Stewart F, Sugiyama N, Toyama J, Toutain A, Vargas AL, Yanazawa M, Zackai EH, Dobyns WB (2004) Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum Mutat 23(2):147–159
Bonneau D, Toutain A, Laquerrière A, Marret S, Saugier-Veber P, Barthez MA, Radi S, Biran-Mucignat V, Rodriguez D, Gélot A (2002) X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): clinical, magnetic resonance imaging, and neuropathological findings. Ann Neurol 51(3):340–349
Okazaki S, Ohsawa M, Kuki I, Kawawaki H, Koriyama T, Ri S, Ichiba H, Hai E, Inoue T, Nakamura H, Goto Y, Tomiwa K, Yamano T, Kitamura K, Itoh M (2008) Aristaless-related homeobox gene disruption leads to abnormal distribution of GABAergic interneurons in human neocortex: evidence based on a case of X-linked lissencephaly with abnormal genitalia (XLAG). Acta Neuropathol 116(4):453–462
Jissendi-Tchofo P, Kara S, Barkovich AJ (2009) Midbrain-hindbrain involvement in lissencephalies. Neurology 72(5):410–418
Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K (2002) Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet 32(3):359–369
Colombo E, Galli R, Cossu G, Gécz J, Broccoli V (2004) Mouse orthologue of ARX, a gene mutated in several X-linked forms of mental retardation and epilepsy, is a marker of adult neural stem cells and forebrain GABAergic neurons. Dev Dyn 231(3):631–639
Wang B, Long JE, Flandin P, Pla R, Waclaw RR, Campbell K, Rubenstein JL (2013) Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol 521(7):1561–1584
Colombo E, Collombat P, Colasante G, Bianchi M, Long J, Mansouri A, Rubenstein JL, Broccoli V (2007) Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci 27(17):4786–4798
Colasante G, Sessa A, Crispi S, Calogero R, Mansouri A, Collombat P, Broccoli V (2009) Arx acts as a regional key selector gene in the ventral telencephalon mainly through its transcriptional repression activity. Dev Biol 334(1):59–71
Yun K, Potter S, Rubenstein JL (2001) Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128(2):193–205
Trzesniak C, Kempton MJ, Busatto GF, de Oliveira IR, Galvão-de Almeida A, Kambeitz J, Ferrari MC, Filho AS, Chagas MH, Zuardi AW, Hallak JE, McGuire PK, Crippa JA (2012) Adhesio interthalamica alterations in schizophrenia spectrum disorders: a systematic review and meta-analysis. Psychol Med 42(12):2523–2534
Olry R, Haines DE (2005) Interthalamic adhesion: scruples about calling a spade a spade? J Hist Neurosci 14(2):116–118
Samra KA, Cooper IS (1968) Radiology of the massa intermedia. Radiology 91(6):1124–1128
Miller E, Widjaja E, Blaser S, Dennis M, Raybaud C (2008) The old and the new: supratentorial MR findings in Chiari II malformation. Childs Nerv Syst 24(5):563–575
Cheng S, Tan K, Bilston LE (2010) The effects of the interthalamic adhesion position on cerebrospinal fluid dynamics in the cerebral ventricles. J Biomech 43(3):579–582
Basel-Vanagaite L, Raas-Rotchild A, Kornreich L, Har-Zahav A, Yeshaya J, Latarowski V, Lerer I, Dobyns WB, Shohat M (2010) Familial hydrocephalus with normal cognition and distinctive radiological features. Am J Med Genet A 152A(11):2743–2748
De France I, Saada P, Jouannic JM, Tantau J, Martinovic J, Encha-Razavi F (2002) Ultrasonographic and pathological correlation in a fetal intracranial cyst: a case of “diencephalo-synapsis”. J Gynecol Obstet Biol Reprod (Paris) 31(6):600–603
Cagneaux M, Vasiljevic A, Massoud M, Allias F, Massardier J, Gaucherand P, Guibaud L (2013) Severe second-trimester obstructive ventriculomegaly related to disorders of diencephalic, mesencephalic and rhombencephalic differentiation. Ultrasound Obstet Gynecol 42(5):596–602
Whitehead MT, Vezina G (2014) Interhypothalamic adhesion: a series of 13 cases. AJNR Am J Neuroradiol 35(10):2002–2006
Acknowledgments
We are grateful to the patients and families for participating in the study. We thank Claudia Mancini for MRI technical support and Serena Stornello for nursing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this human study has been approved by our Institutional Review Board and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Due to the retrospective nature of this study, patient consent was waived.
Conflict of interest
We declare that we have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
On-line Resource 1: Table 2
Neuroradiological findings of patients with diencephalic-mesencephalic junction abnormalities (PDF 81.6 kb)
On-line Resource 2
Scatterplots of MH measurements for age categories in patients with DMJ anomalies at last MRI examination and 417 healthy controls. Anteroposterior (AP) diameter of the midbrain (A), pons (B), medulla (C), midbrain to pons (M/P) ratio of AP diameters (D), cranio-caudal (CC) diameter of the midbrain (E), pons (F), medulla (G), midbrain to pons (M/P) ratio of CC diameters (H), CC diameter of the vermis (I), vermian AP diameter (L), transverse cerebellar diameter (M) are shown. (PDF 145 kb)
Rights and permissions
About this article
Cite this article
Severino, M., Tortora, D., Pistorio, A. et al. Expanding the spectrum of congenital anomalies of the diencephalic–mesencephalic junction. Neuroradiology 58, 33–44 (2016). https://doi.org/10.1007/s00234-015-1601-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1601-x