Abstract
Introduction
The aim of this study is to investigate whether early changes in tumor volume and perfusion measurements derived from dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) may predict response to antiangiogenic therapy in recurrent high-grade gliomas.
Methods
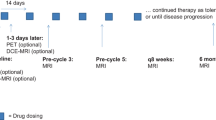
Twenty-seven patients who received bevacizumab every 3 weeks were enrolled in the study. For each patient, three MRI scans were performed: at baseline, after the first dose, and after the fourth dose of bevacizumab. The entire tumor volume (Vtot), as well as contrast-enhanced and noncontrast-enhanced tumor subvolumes (VCE-T1 and VNON-CE-T1, respectively) were outlined using post-contrast T1-weighted images as a guide for the tumor location. Histogram analysis of normalized IAUGC (nIAUGC) and transfer constant Ktrans maps were performed. Each patient was classified as a responder patient if he/she had a partial response or a stable disease or as a nonresponder patient if he/she had progressive disease.
Results
Responding patients showed a larger reduction in VNON-CE-T1 after a single dose, compared to nonresponding patients. Tumor subvolumes with increased values of nIAUGC and Ktrans, after a single dose, significantly differed between responders and nonresponders. The radiological response was found to be significantly associated to the clinical outcome. After a single dose, Vtot was predictive of overall survival (OS), while VCE-T1 showed a tendency of correlation with OS.
Conclusion
Tumor subvolumes with increased nIAUGC and Ktrans showed the potential for improving the diagnostic accuracy of DCE. Early assessments of the entire tumor volume, including necrotic areas, may provide complementary information of tumor behavior in response to anti-VEGF therapies and is worth further investigation.





Similar content being viewed by others
References
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358:2039–2049
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Khasraw M, Ameratunga M, Grommes C (2014) Bevacizumab for the treatment of high-grade glioma: an update after phase III trials. Expert Opin Biol Ther 14:729–740
Jalali S, Chung C, Foltz W et al (2014) MRI identify the differential response of glioblastomamultiforme to anti-angiogenic-therapy. Neuro Oncol 16:868–879
de Groot JF, Fuller G, Kumar AJ et al (2010) Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in human and mice. Neuro Oncol 12:233–242
Macdonald DR et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Leu K, Pope WB, Cloughesy TF et al (2013) Imaging biomarkers for antiangiogenic therapy in malignant gliomas. CNS Oncol 2:33–47
Sawlani RN, Raizer J, Horowitz SW et al (2010) Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging-pilot study. Radiology 255:622–628
Essock-Burns E, Lupo JM, Cha S et al (2011) Assessment of perfusion MRI-derived parameters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma. Neuro Oncol 13:119–131
Schmainda KM, Prah M, Connelly J et al (2014) Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol 16:880–888
Sorensen AG, Batchelor TT, Zhang WT et al (2009) A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranibin recurrent glioblastoma patients. Cancer Res 69:5296–5300
Ellingson BM, Sahebjam S, Kim HJ et al (2014) Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am J Neuroradiol 35:673–679
Nowosielski M, Recheis W, Goebel G et al (2011) ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53:291–302
Pope WB, Kim HJ, Huo J et al (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252:182–189
Zhang W, Kreisl TN, Solomon J (2009) Acute effects of bevacizumab on glioblastoma vascularity assessed with DCE-MRI and relation to patient survival. Proc. Intl. Soc. Mag. Reson. Med. http://cds.ismrm.org/protected/09MProceedings/files/00282.pdf
Kickingereder P, Wiestler B, Graf M et al (2015) Evaluation of dynamic contrast-enhanced MRI derived microvascular permeability in recurrent glioblastoma treated with bevacizumab. J Neurooncol 121:373–380
Verhoeff JJ, Lavini C, van Linde ME et al (2010) Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol 21:1723–1727
Hattingen E, Jurcoane A, Bähr O et al (2011) Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro Oncol 13:1349–1363
Vidiri A, Pace A, Fabi A et al (2012) Early perfusion changes in patients with recurrent high-grade brain tumour treated with Bevacizumab: preliminary results by a quantitative evaluations. J Exp Clin Cancer Res 31:33
Schwarzenberg J, Czernin J, Cloughesy TF et al (2014) Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res 20:3550–3559
Ono T, Sasajima T, Doi Y et al (2015) Amino acid PET tracers are reliable markers of treatment responses to single-agent or combination therapies including temozolomide, interferon-β, and/or bevacizumab for glioblastoma. Nucl Med Biol. doi:10.1016/j.nucmedbio.2015.01.008
O’Connor JP, Jackson A, Parker GJ, Robert C, Jayson GC (2012) Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 9:167–177
Jia Z, Geng D, Xie T, Zhang J, Liu Y (2012) Quantitative analysis of neovascular permeability in glioma by dynamic contrast-enhanced MR imaging. J Clin Neurosci 19:820–823
Millis SJ, Soh C, O’Connor JP et al (2010) Enhancing fraction in glioma and its relationship to the tumoral vascular microenvironment: a dynamic contrast-enhanced MR imaging study. AJNR Am J Neuroradiol 31:726–731
Yopp AC, Schwartz LH, Kemeny N et al (2011) Antiangiogenic therapy for primary liver cancer: correlation of changes in dynamic contrast-enhanced magnetic resonance imaging with tissue hypoxia markers and clinical response. Ann Surg Oncol 18:2192–2199
Nardo G, Favaro E, Curtarello M et al (2011) Glycolytic phenotype and AMP kinase modify the pathologic response of tumor xenografts to VEGF neutralization. Cancer Res 71:4214–4225
Toft PS, Brix G, Bucley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341
Artzi M, Blumenthal DT, Bokstein F et al (2015) Classification of tumor area using combined DCE and DSC MRI in patients with glioblastoma. J Neurooncol 121:349–357
Hartigan JA, Wong MA (1979) Algorithm AS136: a k-means clustering algorithm. Appl Statist 28:100–108
Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB (2011) Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol 13:401–409
Huang RY, Rahman R, Hamdan A et al (2013) Recurrent glioblastoma: volumetric assessment and stratification of patient survival with early post treatment magnetic resonance imaging in patients treated with bevacizumab. Cancer 119:3479–3488
Masunaga S, Liu Y, Tanaka H et al (2011) Reducing intratumour acute hypoxia through bevacizumab treatment, referring to the response of quiescent tumour cells and metastatic potential. Br J Radiol 84:1131–1138
Goh V, Ng QS, Miles K (2012) Computed tomography perfusion imaging for therapeutic assessment: has it come of age as a biomarker in oncology? Invest Radiol 47:2–4
Rose CJ, Mills S, O'Connor JP et al (2007) Quantifying heterogeneity in dynamic contrast-enhanced MRI parameter maps. Med Image Comput Comput Assist Interv 10:376–384
Del Vecchio M, Mortarini R, Canova S et al (2010) Bevacizumab plus fotemustine as first-line treatment in metastatic melanoma patients: clinical activity and modulation of angiogenesis and lymphangiogenesis factors. Clin Cancer Res 16:5862–5872
Soffietti R, Trevisan E, Bertero L, Cassoni P et al (2014) Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology). J Neurooncol 116:533–541
Vaccaro V, Fabi A, Vidiri A et al (2014) Activity and safety of bevacizumab plus fotemustine for recurrent malignant gliomas. Biomed Res Int. doi:10.1155/2014/351252
Taal W, Oosterkamp HM, Walenkamp AM et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953
Acknowledgments
The authors are indebted to the GE Advanced Clinical Education Specialist Lorenzo Viarengo for his contribution to the MR sequence optimization and to Gaetano Fetonti and Michele Farella for their continued technical assistance.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the our Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
FP and SM contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
ESM 2
(DOC 36 kb)
ESM 3
(DOC 29 kb)
Figure 6
Averages of the differential histograms of the normalized initial area under the gadolinium concentration curve (nIAUGC) inside the entire lesion for the patient groups with progressive disease or partial response/stable disease at baseline and after the first and fourth doses of bevacizumab. (GIF 100 kb)
Figure 7
Averages of the differential histograms of the transfer constant (Ktrans) inside the entire lesion for the patient groups with progressive disease or partial response/stable disease at baseline and after the first and fourth doses of bevacizumab. (GIF 89 kb)
Rights and permissions
About this article
Cite this article
Piludu, F., Marzi, S., Pace, A. et al. Early biomarkers from dynamic contrast-enhanced magnetic resonance imaging to predict the response to antiangiogenic therapy in high-grade gliomas. Neuroradiology 57, 1269–1280 (2015). https://doi.org/10.1007/s00234-015-1582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1582-9