Abstract
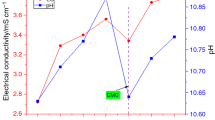
The effect of time dependent nanoclusters morphology on heat conduction mechanism of a nanofluid has been presented over here. The time dependent growth of nanoclusters of TiO2 nanoparticles (size 25–30 nm, volume fraction of 0.05%) along with surfactant (SDBS) in water (DI), has been investigated. A detailed report on the size distribution of nanoparticles while in suspension for an actual volume fraction of the nanoparticles at a particular instant of time is presented. The different pH values (2.92–11.62) along with surfactant (SDBS, 1:1 by wt.) have been used to investigate the suspension stabilities of different smaples of the nanofluid. The suspension stabilities have been evaluated by measuring the zeta potential and stability ratios of TiO2–H2O nanofluid. A quantitative analysis on the role of nanoparticles presented in the form of dead-ends and backbone chains, has been carried out. The effect of liquid layering of the water present inside the characteristic dimension of a nanocluster is taken into account to determine the effective thermal conductivity of a nanofluid. The so, obtained experimental and theoretical results of thermal conductivities have been correlated and found to be predicted well with a new developed model and with an accuracy level of +2.82 to −1.5%. Whereas, the predictions from other fundamental models and empirical correlations are found to be vary with an error from +3 to +8.1% on one side and from −4.87 to −12.62% on other side, in the volume fraction from 0.01 to 0.12%, when analysed.












Similar content being viewed by others
Abbreviations
- a :
-
Average hydrodynamic size (diameter) of the particle (nm)
- A 132 :
-
Hamaker’s constant (J)
- B (x):
-
Parameter represents the hydration interaction among the nanoparticles and the basefluid
- C :
-
Aggregate density of the nanoparticles per unit cluster
- d l :
-
Chemical dimension of a cluster
- d f :
-
Fractal dimension of a cluster
- E R :
-
Electrostatic repulsion (J)
- E A :
-
Vander Waals attraction (J)
- E tot :
-
Total interaction energy (J)
- I :
-
Concentration of ions in aqueous medium
- K eff /K nf :
-
Thermal conductivity of nanofluid (W/mK)
- K exp :
-
Experimental thermal conductivity (W/mK)
- K l :
-
Thermal conductivity of bulk base fluid (W/mK)
- K p :
-
Thermal conductivity of nanopowder (W/mK)
- K f :
-
Thermal conductivity of the fluid present inside the nanocluster (W/mK)
- K f1 :
-
Thermal conductivity enhancement of the fluid present inside the nanocluster (W/mK)
- K db :
-
Thermal conductivity enhancement inside the nanocluster due to deadend nanoparticles (W/mK)
- K adb :
-
Cluster thermal conductivity due to presence of nanoparticles as dead-ends and backbone chains (W/mK)
- K B :
-
Boltzmann constant (1.3807 × 10−23 J/K)
- N :
-
Average number of nanoparticles per cluster
- N b :
-
Number of nanoparticles belongs to backbone chains
- p :
-
Aspect ratio
- pH:
-
pH level of the nanofluid
- R BL :
-
Resistance due to thermal boundary layer (m2 K/W)
- R g :
-
Cluster characteristic dimension (nm)
- rp :
-
Average radius of the nanoparticle (nm)
- S:
-
Surfactant amount (mg)
- t p :
-
Aggregation time constant (s)
- T :
-
Temperature (°C)
- V a :
-
Volume of a cluster
- V na :
-
Volume of the nanoparticles per cluster
- W :
-
Stability ratio
- Wt.:
-
Weight (mg)
- x :
-
Particle surface to surface distance (nm)
- DI:
-
De-ionized water
- DLS:
-
Dynamic light scattering
- DLCA:
-
Diffusion limited colloidal aggregation
- M-G:
-
Model Maxwell Garnet Model
- SSA:
-
Specific surface area
- XRD:
-
X-rays diffraction
- SDBS:
-
Sodium dodecyl benzene sulphonate
- t :
-
Time
- TEM:
-
Transmission electronic microscope
- α :
-
A constant (13.58 × 1020 s/m3)
- α nf :
-
Thermal diffusivity (m2/s)
- ρ nf :
-
Density of nanofluid (kg/m3)
- ρ f :
-
Density of base fluid (kg/m3)
- ρ TiO2 :
-
Density of TiO2 nanoparticles(kg/m3)
- μ :
-
Viscosity of basefluid (Pas)
- φ p/ φ :
-
Volume fraction of the primary nanoparticles in the basefluid (%)
- φ a :
-
Volume fraction of the nanoparticles in an aggregate
- φ bp :
-
Volume fraction of backbone particles in the aggregate
- φ dp :
-
Volume fraction of the particles belonging to deadends
- φ f1 :
-
Volume fraction of fluid elements present in the aggregate
- φ a t :
-
Total Volume fraction of the aggregates present in the base fluid (%)
- ε r :
-
Relative dielectric constant of the liquid
- ε 0 :
-
Dielectric constant of free space
- ζ :
-
Zeta potential (mV)
- Λ :
-
Debye parameter
- Π :
-
Pi (3.14159)
References
Lee S, Choi SUS, Li S, Eastman JA (1999) Measuring thermal conductivity of fluids containing nanoparticles. J Heat Transf 121(2):280–289
Xie HQ, Wang JC, Xi TG, Liu Y, Ai F, Wu QR (2002) Thermal conductivity enhancement of suspensions containing nanosized alumia particles. Appl Phys 91(7):4568–4572
Masuda H, Ebata A, Teramae K, Hishinuma N (1993) Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Netsu Bussei 7(4):227–233
Das SK, Putra N, Thiesen P, Roetzel W (2003) Temperature dependence of thermal conductivity enhancement for nanofluids. J Heat Transf 125:567–574
Esfe MH, Saedodin S, Mahian O, Saedodin S (2014) Thermal conductivity of Al2O3/water nanofluids: measurement, correlation, sensitivity analysis and comparisons with literature reports. J Therm Anal Calorim 117:675–681
Jiang W, Ding G, Peng H, Haitao H (2010) Modelling of nanoparticles’ aggregation and sedimentation in nanofluid. Curr Appl Phys 10(3):934–941
Prasher R, Phelen PE, Bhattacharya P (2006) Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions (nanofluid). Nanoletter 6:1529–1534
Timofeeva EV, Gavrilov AN, McCloskey JM, Tolmachev YV, Sprunt S, Lopatina LM, Selinger JV (2007) Thermal conductivity and particle agglomeration in alumina nanofluids: experiment and theory. J Phys Rev E 76:061703-1–061703-16
Lin CY, Wang JC, Chen TC (2011) Analysis of suspension and heat transfer characteristics of Al2O3 nanofluids prepared through ultrasonic vibration. J Appl Energy 88:4527–4533
Chanderasekar M, Suresh S (2009) A review on the mechanisms of heat transport in nanofluids. Heat Transf Eng 30(14):1136–1150
Wang X, Xu X, Choi SUS (1999) Thermal conductivity of nanoparticle-fluid mixture. J Thermophys Heat Transf 13(4):474–480
Yu W, Choi SUS (2003) The role of interfacial layers in the enhanced thermal conductivity of nanofluids: a renovated Maxwell model. J Nanoparticle Res 5:167–171
Gao JW, Zheng RT, Ohtani H, Zhu DS, Chen G (2009) Experimental investigation of heat conduction mechanisms in nanofluids: clue on clustering. Nanoletter 9:4128–4132
Ozerinc S, Kakac S, Guvenc AY (2010) Enhanced thermal conductivity of nanofluids: a state-of-the-art review. Microfluid Nanofluid 8:145–170
Mishra A, Kundan L, Mallick SS (2014) Modeling thermal conductivity for alumina-water nanofluids. Part Sci Technol 32(3):319–326
Karthikeyan NR, Philip J, Raj B (2008) Effect of clustering on the thermal conductivity of nanofluids. Mater Chem Phys 109:50–55
Shih WH, Shih WY, Kim SI, Liu J, Aksay A (1990) Scaling behavior of the elastic properties of colloidal gels. Phys Rev A 42:4772–4779
Evans W, Prasher R, Fish J, Meakin P, Phelan PE, Keblinski P (2008) Effect of aggregation and interfacial thermal resistance on thermal conductivity of nanocomposites and colloidal nanofluids. Int J Heat Mass Transf 51:1431–1438
Xuan Y, Li Q, Hu W (2003) Aggregation structure and thermal conductivity of nanofluids. AIChE J 49:1038–1043
Philip J, Shima PD, Raj B (2008) Evidence for enhanced thermal conduction through percolating structures in nanofluids. Nanotechnology 19:305706
Pang C, Lee JW, Kang YT (2016) Enhanced thermal conductivity of nanofluids by nanoconvection and percolation network. Heat Mass Transf 52:511–520
Longo G, Zilio AC (2011) experimental measurement of thermophysical properties of oxide–water nano- fluids down to ice-point. Exp Thermal Fluid Sci 35:1313–1324
Longo G, Zilio AC, Ceseracciu E, Reggiani M (2012) Application of artificial neural network (ANN) for the prediction of thermal conductivity of oxide–water nanofluids. Nano Energy 1:290–296
Hays A, Marsh CP, Alvarado J, Franks R (2006) The effect of nanoparticle agglomeration on enhanced nanofluidic thermal conductivity. In: Proceedings of international refrigeration and air conditioning conference, Purdue University, vol R132, pp 1–7
Alipour R, Ghoranneviss M, Mirzaee M, Jafari A (2014) The direct effect of interfacial nanolayers on thermal conductivity of nanofluids. Heat Mass Transf 50:1727–1735
Mallick SS, Mishra A, Kundan L (2013) An investigation into modelling thermal conductivity for alumina-water nanofluids. Powder Technol 233:234–244
Maxwell JC (1873) A treatise on electricity and magnetism, 2nd edn. Clarendon Press, Oxford
Hamilton RL, Crosser OK (1962) Thermal conductivity of heterogeneous two-component systems. I EC Fundam 1:182–191
Hui PM, Zhang XA, Markworth J, Stroud D (1999) Thermal conductivity of graded composites: numerical simulations and an effective medium approximation. J Mater Sci 34:5497–5503
Rizvi IH, Jain A, Ghosh SK, Mukherjee PS (2013) Mathematical modelling of thermal conductivity for nanofluid considering interfacial nano-layer. Heat Mass Transf 49:595–600
Kumar DH, Patel HE, Rajeev Kumar VR, Sundararajan T, Pradeep T, Das SK (2004) Model for heat conduction in nanofluids. Phys Rev Lett 93:144301
Prasher R, Bhattacharya P, Phelan PE (2005) Thermal conductivity of nanoscale colloidal solutions (nanofluids). Phys Rev Lett 94(2):025901
Jang SP, Choi SUS (2004) Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl Phys Lett 84:4316–4318
Bhattacharya P, Saha SK, Yadav A, Phelan PE, Prasher RS (2004) Brownian dynamics simulation to determine the effective thermal conductivity of nanofluids. J Appl Phys 95:6492–6494
Koo J, Kleinstreuer C (2004) A new thermal conductivity models for nanofluids. J Nanopart Res 6:577–588
Mukherjee S, Mishra PCh, Prashar SKS, Chaudhuri P (2016) Role of temperature on thermal conductivity of nanofluids: a brief literature review. Heat Mass Transf. doi:10.1007/s00231-016-1753-1
Zhu D, Li X, Wang N, Wang X, Gao J, Li H (2009) Dispersion behavior and thermal conductivity characteristics of Al2O3–H2O nanofluids. Curr Appl Phys 9:131–139
Das SK, Choi SUS, Yu W, Pardeep T (2008) Nanofluids science and technology. Wiley, New York
Xie HQ, Lee H, Youn W, Choi M (2003) Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J Appl Phys 94:4967–4971
Lee D, Kim JW, Kim BG (2006) A new parameter to control heat transport in nanofluids: surface charge state of the particles in suspension. J Phys Chem B 110:4323–4328
Xie HQ, Wang JC, Xi TG, Liu Y, Ai F, Wu QR (2007) Thermal conductivity enhancement of suspensions containing nanosized alumia particles. Appl Phys 91(7):4568–4572
Putra N, Roetzel W, Das SK (2003) Natural convection of nano-fluids. Heat Mass Transf 39:775–784
Jang SP, Choi SUS (2004) Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl Phys Lett 84(21):4316–4318
Wen DS, Ding YL (2004) Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. Int J Heat Mass Transf 47(24):5181–5188
Zhang X, Gu H, Fujii M (2006) Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. J Appl Phys 100(4):044325
Russel WB, Saville DA, Schowalter WR (1989) Colloidal dispersions. Cambridge University Press, New York
Hunter RJ (2001) Foundations of colloid science. Clarendon Press, Oxford
Hidalgo-Alvarez R, Martin A, Fernandez A, Bastos D, Martinez F (1996) Electrokinetic properties, colloidal stability and aggregation kinetics of polymer colloids. Adv Colloid Interface Sci 67:1–118
Hemker DJ, Frank CW (1990) Dynamic light scattering studies of fractal aggregation of poly (methacrylic acid) and poly (ethylene glycol). Macromolecules 23:4404–4410
Lin MY, Lindsay HM, Weitz DA, Ball RC, Klein R, Meakin P (1989) Universality in colloid aggregation. Nature 339:360–362
De Rooij R, Potanin AA, Van den Ende D, Mellema J (1993) Steady shear viscosity of weakly aggregating polystyrene latex dispersions. J Chem Phys 99:1993–9213
Lin MY, Lindsay HM, Weitz DA, Ball RC, Klein R, Meakin P (1990) Universal diffusion-limited colloid aggregation. J Phys Condens Matter 2:3093–3113
Wang BX, Zhou LP, Peng XF (2013) A fractal model for predicting the effective thermal conductivity of liquid with suspension of nanoparticles. Int J Heat Mass Transf 46(14):2665–2672
Waite TD, Cleaver JK, Beattie JK (2001) Aggregation kinetics and fractal structure of γ-alumina assemblages. J Colloid Interface Sci 241:333–339
Bruggeman DAG (1935) Berechnung verschiedener physikalischer konstanten von heterogenen substanzen, i. dielektrizitatskonstanten und leitfahigkeiten der mischkorper aus isotropen substanzen. Annal Phys Leipz 24:636–679
Holthoff H, Egelhaaf SU, Borkovec M, Schurtenberger P, Sticher H (1996) Coagulation rate measurements of colloidal particles by simultaneous static and dynamic light scattering. Langmuir 12:5541–5549
Carpineti M, Giglio M (1993) Aggregation phenomena. Adv Colloid Interface Sci 46:73–90
Potanin AA, De Rooij R, Van den Ende D, Mellema J (1995) Microrheological modeling of weakly aggregated dispersions. J Chem Phys 102:5845
Kallay N, Zalac S (2001) Introduction of the surface complexation model into the theory of colloid stability. Croat Chem Acta 74(3):479–497
Bergstrom L (1997) Hamaker constants of inorganic materials. Adv Colloid Interface Sci 70:125–169
Hiemenz PC (1997) Principles of colloid and surface chemistry. Marcel Dekker, New York
Honig EP, Roebersen GJ, Wiersema PH (1971) Effect of hydrodynamic interaction on the coagulation rate of hydrophobic colloids. J Colloid Interface Sci 36(1):97–109
Nan CW, Birringer R, Clarke DR, Gleiter H (1997) Effective thermal conductivity of particulate composites with interfacial thermal resistance. J Appl Phys 81:6692–6699
Ge ZB, Cahill DG, Braun PV (2006) Thermal conductance of hydrophilic and hydrophobic interfaces. Phys Rev Lett 96:186101–186104
Schmidt AJ, Alper JD, Chiesa M, Chen G, Das SK, Hamad-Schifferli K (2008) Probing the gold nanorod-ligand-solvent interface by plasmonic absorption and thermal decay. J Phys Chem C 112:13320–13323
Bailar JC (1973) Comprehensive inorganic chemistry. Pergamon, Oxford
Patnaik P (2002) Handbook of inorganic chemicals. McGraw-Hill, New York
Li X, Li F, Zhu DS, Wang XJ (2007) evaluation on dispersion behavior of the aqueous copper nano- suspensions. J Colloid Interface Sci 310(2):456–463
Jeffrey D (1973) Conduction through a random suspension of spheres. R Soc Lond Ser A 335(1602):355–367
Pak BC, Choi YI (1998) Hydraulic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Transf 11:151–170
Li C, Peterson GP (2006) Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticles suspensions (nanofluids). J Appl Phys 99(8):084314
Teng TP, Hung YH, Teng TC, Moa HE, Hsu HG (2010) The effect of alumina/water nanofluid particle size on thermal conductivity. J Appl Therm Eng 30:2213–2218
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kundan, L., Mallick, S.S. & Pal, B. Effect of time dependent nanoclusters morphology on the thermal conductivity and heat transport mechanism of TiO2 based nanofluid. Heat Mass Transfer 53, 1873–1892 (2017). https://doi.org/10.1007/s00231-016-1945-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-016-1945-8