Abstract
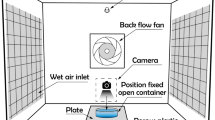
Desalination by freezing is of great prospect due to its low energy consumption, little pollution, freedom of corrosion and ease of scaling. A novel freezing desalination method driven by humidity difference between air flow and liquid surface is proposed and verified in this paper. In order to reveal its mechanism, an unsteady-state thermodynamics model was established to simulate icing movement on liquid surface according to heat and mass balance. Effects of humidity difference, airflow velocity and mass diffusion coefficient on icing development were analyzed through theory analysis and experiments. It was found that the larger humidity difference and larger airflow velocity had evident effects on the liquid evaporation-freezing. Meanwhile, fast mass diffusion between mediums was also beneficial to this freezing process.













Similar content being viewed by others
Abbreviations
- u :
-
Velocity along z-direction (m/s)
- δ :
-
Boundary layer thickness (m)
- D :
-
Mass diffusion coefficient (m2/s)
- q :
-
Heat quantity (J)
- S A :
-
Solidification heat of liquid (kJ/kg)
- H A :
-
Latent heat of vaporization (kJ/kg)
- M :
-
Entire evaporation amount (kg)
- y :
-
y-direction
- φ :
-
Absolute humidity (g/(kg dry air))
- K :
-
Proportional coefficient
- ρ :
-
Concentration (kg/m3)
- B :
-
Cross section width of evaporation-freezing chamber (m)
- G :
-
Length of evaporation-freezing chamber (m)
- L :
-
Cross section height of evaporation-freezing chamber (m)
- r :
-
Distance from the air–liquid interface to the center of airflow channel (m)
- w :
-
Air-liquid interface
- N :
-
Nth unit body
- o :
-
Outlet
- U :
-
Turbulent velocity (m/s)
- C :
-
Concentration value of mainstream
- Re :
-
Reynolds number
- I :
-
Amount of ice (kg)
- M NA :
-
Evaporation quantity of the liquid (kg)
- τ :
-
Time (s)
- N :
-
Unit body number
- Z :
-
z-direction
- F :
-
Ice body thickness (m)
- f :
-
The distance of dendrite tip toxaxis (m)
- V :
-
Volume (m3)
- Nf :
-
Airflow mainstream concentration
- i :
-
Inlet
- ice :
-
Ice body
References
Pingli L, Jia M, Lixin X, Shichang W (2005) Recent progress of seawater desalination by freezing. Chem Ind Eng Prog 7:749–753
Khawaji AD, Kutubkhanah IK, Wie J-M (2008) Advances in seawater desalination technologies. Desalination 221:47–69
Georgiadis JG, Ramaswamy M (2005) Magnetic resonance imaging of water freezing in packed beds cooled from below. Int J Heat Mass Transf 48:1064–1075
Hillig WB (1998) Measurement of interfacial free energy for ice/water system. J Cryst Growth 183:463–468
Okawa S, Saito A, Kadoma Y, Kumano H (2010) Study on a method to induce freezing of supercooled liquor using a membrane. Refrigeration 33:1459–1464
Yuan MA, Xiaofeng PENG (2008) Competition phenomena of crystals in directional freezing of aqueous liquors. Chem Ind Eng Soc China J 11:2857–2863
Yunfei D, Shuai Y, Yundan L, Huijun W (2012) Frosting mechanism and suppression on nano/micro-structures hydrophobic surfaces. Chem Ind Eng Soc China J 10:3213–3219
Maykut GA, Untreateiner N (1971) Some results from a time-dependent thermodynamic model of sea icing. J Phys Res 76:1550–1575
Maykut GA (1978) Energy exchange over young sea icing in the central arctic. J Phys Res 83:3646–3658
Cole DM (2001) The microstructure of ice and its influence on mechanical properties. Eng Fract Mech 68:1797–1822
Dickey LC (1996) Evaporation of water from agitated freezing slurries at low pressure. Desalination 104:155–163
Mandri Youssef, Rich Anouar, Mangin Denis, V S et al (2011) Parametric study of the sweating step in the seawater desalination process by indirect freezing. Desalination 269:142–147
Rich A, Mandri Y, Bendaoud N, Mangin D, Abderafi S et al (2010) Freezing desalination of sea water in s static layer crystallizer. Desalin Water Treat 13:120–127
Beier N, Sego D, Donahue R, Biggar K (2007) Laboratory investigation on freeze separation of saline mine waste water. Cold Reg Sci Technol 48:239–247
Wijeysundera NE, Hawlader MNA, Andy CWB, Hossain MK (2004) Ice-slurry production using direct contact heat transfer. Int J Refrig 27:511–519
Hongjun J (1988) Fluid mechanics. Higher Education Press, Beijing
Wang Z, Zhang Y, Zhang H et al (2011) Un-balance concretion theory and technology. China Machine Press, Beijing
Chang W (2008) Studies on flux and fouling in direct contact membrane distillation of seawater. Master’s thesis of Tianjin University, Tianjin
Acknowledgments
The work was supported by Fundamental Research Funds for the Central Universities (No. 2014QNA74).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, P., Zhang, D. & Zhou, G. Study on icing evolution rule in process of liquid evaporation-freezing by humidity difference. Heat Mass Transfer 51, 1537–1547 (2015). https://doi.org/10.1007/s00231-015-1519-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-015-1519-1