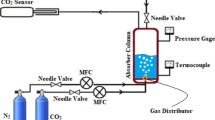
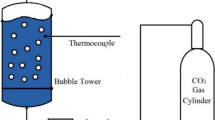
Abstract
Magnetic nanofluids have been used for absorption of CO2 in a packed column in the presence of magnetic field. Adding nanoparticles into the solvent enhances mass transfer characteristics. The optimum concentrations of Fe3O4/water and NiO/water nanofluids are 0.005, and 0.01 % respectively, and the maximum enhancement of mass transfer rate in comparison with pure water is 12 and 9.5 % respectively. The magnetic field showed positive effect on the CO2 absorption performance.












Similar content being viewed by others
Abbreviations
- a:
-
Specific surface area of packing (m2 m−3)
- C:
-
Constant in Eq. 4
- C:
-
Concentration (mol l−1)
- d:
-
Diameter (m)
- D:
-
Diffusion coefficient (m2 s−1)
- Fr:
-
Froude number
- g:
-
Gravitational acceleration (ms−2)
- hl :
-
Column holdup (m3 m−3)
- Kla:
-
Mass transfer coefficient (s−1)
- NA :
-
Flux of mass transfer (mol m−3 s−1)
- Q:
-
Fluid flow rate (l s−1)
- Re:
-
Reynolds number
- S:
-
Tower cross section (m2)
- \( \overline{U} \) :
-
Mean effective velocity (ms−1)
- U:
-
Velocity (ms−1)
- We:
-
Weber number
- Z:
-
Height of the tower (m)
- µ:
-
Viscosity (kg s−1)
- ρ:
-
Density (kg m−3)
- *:
-
Saturation
- ave:
-
Average
- bs:
-
Base fluid
- eff:
-
Effective
- i:
-
Inlet
- l:
-
Liquid
- mf:
-
Magnetic field
- nf:
-
Nanofluid
- o:
-
Outlet
References
Nik Hisyamudin BMN, Yokoyama S, Umemoto M (2009) Absorption of CO2 in EAF reducing slag from stainless steel making process by wet grinding. World Acad Sci Eng Technol 32:611–615
Kang YT, Kunugi Y, Kashiwagi T (2000) Review of advancedabsorption cycles: performance improvement and temperaturelift enhancement. Int J Refrig 23:388–411
Daiguji H, Hihara E, Saito T (1997) Mechanism of absorption enhancement by surfactant. Int J Heat Mass Transf 40:1743–1752
Kashiwagi T (1988) Basic mechanism of absorption, heat and mass transfer enhancement by the marangoni effect. Int Energy Agency Heat Pump Center 6:2–6
Veilleux J, Coulombe S (2011) A dispersion model of enhanced mass diffusion in nanofluids. Chem Eng Sci 66(11):2377–2384
Choi SUS (1995) Enhancing thermal conductivity of fluids with nanoparticles. ASME Fluids Eng Div 231:99–105
Kim JK, Jung JY, Kang YT (2007) Absorption performance enhancement by nano-particles and chemical surfactants in binary nanofluids. Int J Refrig 30:50–57
Ma X, Su F, Chen J, Bai T, Han Z (2009) Enhancement of bubble absorption process using a CNTs-ammonia binary nanofluid. Int Commun Heat Mass Transf 36:657–660
Kim W, Kang HU, Jung K, Kim SH (2008) Synthesis of silica nanofluid and application to CO2 absorption. Sep Sci Technol 4311:3036–3055
Samadi Z, Haghshenasfard M, Moheb A (2011) Effect of nanofluid on mass transfer coefficient of CO2 in a wetted wall column. Int Chem Eng Cong & Exihibition Kish Island, Iran
Lee JW, Jung JY, Lee SG, Kang YT (2011) CO2 bubble absorption enhancement in methanol-based nanofluids. Int J Refrig 34:1727–1733
Pineda IT, Lee JW, Jung I, Kang YT (2012) CO2 absorption enhancement by methanol-based Al2O3 and SiO2 nanofluids in a tray column absorber. Int J Refrig 35:1402–1409
Komati S, Suresh A (2008) CO2 absorption into amine solutions: a novel strategy for intensification based on the addition of ferrofluids. J Chem Technol Biotech 83:1094–1100
Niu XF, Du K, Xiao F (2010) Experimental study on ammonia–water falling film absorption in external magnetic fields. Int J Refrig 33:686–694
Wu WD, Liu G, Chen SX, Zhang H (2013) Nanoferrofluid addition enhances ammonia/water bubble absorption in an external magnetic field. Energy Build 57:268–277
Metha D, Hawley MC (1969) Wall effect in packed columns. Ind Eng Chem Process Des Dev 8:280–282
Joubert R, Willie N (2013) Trickle flow liquid–solid mass transfer and wetting efficiency in small diameter columns. Can J Chem Eng 91(3):441–447
Templeman JJ, Porter KE (1965) Experimental determination of wall flow in packed columns. Chem Eng Sci 21:1139–1140
Billet R, Schultes M (1999) Prediction of mass transfer columns with dumped and arranged packings: updated summary of the calculation method of Billet and Schultes. Chem Eng Res Des 77(6):498–504
Mamaliga I, Sidor D, Condurat C, Tudose ETI (2014) Hydrodynamics and mass transfer coefficients for a modified Raschig ring packed column. Heat Mass Transf. doi:10.1007/s00231-014-1324-2
Fang X, Xuan Y, Li Q (2009) Experimental investigation on enhanced mass transfer in nanofluids. Appl Phys Lett 95(20):203108
Veilleux J, Coulombe S (2011) A dispersion model of enhanced mass diffusion in nanofluids. Chem Eng Sci 66(11):2377–2384
Nagy E, Feczko T, Koroknai B (2007) Enhancement of oxygen mass transfer rate in the presence of nanosized particles. Chem Eng Sci 62(24):7391–7398
Rosu M, Marlina A, Kaya A, Schumpe A (2007) A Surfactant adsorption onto activated carbon and its effect on absorption with chemical reaction. Chem Eng Sci 62:7336–7343
Kim JK, Jung JY, Kang YT (2006) The effect of nano-particles on the bubble absorption performance in a binary nanofluid. Int J Refrig 29:22–29
Kars RL, Best RJ, Drinkenburg AAH (1979) The sorption of propane in slurries of active carbon in water. Chem Eng J 17:201–210
Alper E, Wichtendahl B, Deckwer WD (1980) Gas Absorption mechanism in catalytic slurry reactors. Chem Eng Sci 35:217–222
Taylor R, Coulombe S, Otanicar T, Phelan P, Gunawan A (2013) Small particles, big impacts: a review of the diverse applications of nanofluids. J Appl Phys 113:011301
Gerardi C, Cory D, Buongiomo J, Hu LW, McKrell T (2009) Nuclear magnetic resonance-based study of ordered layering on the surface of alumina nanoparticles in water. Appl Phys Lett 95:253104
Kim WG, Kang HU, Jung KM, Kim SH (2008) Synthesis of silica nanofluid and application to CO2 absorption. Sep Sci Technol 43(11–12):3036–3055
Cussler EL (2009) Diffusion: Mass transfer in fluid systems. Cambridge Series in Chemical Engineering. Cambridge University Press, London
Chantrell RW, Bradbury A, Popplewell J, Charles SW (1982) Agglomerate formation in a magnetic fluid. J Appl Phys 53(3):2742
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salimi, J., Haghshenasfard, M. & Etemad, S.G. CO2 absorption in nanofluids in a randomly packed column equipped with magnetic field. Heat Mass Transfer 51, 621–629 (2015). https://doi.org/10.1007/s00231-014-1439-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-014-1439-5