Abstract
Purpose
Mycophenolate mofetil (MMF), a prodrug of the immunosuppressive agent mycophenolic acid (MPA), is widely used for prophylaxis of solid organ transplant rejection. MPA is primarily metabolized to 7-O-mycophenolic acid glucuronide (MPAG), an inactive metabolite that undergoes enterohepatic recirculation (EHC). This study assessed ethnic differences in the pharmacokinetics (PK) of MPA and MPAG between healthy Chinese and Caucasian subjects using population PK analysis.
Methods
Data were pooled from 132 healthy subjects (80 Chinese, 52 Caucasians) in eight clinical studies in which MMF was administered in a single oral dose. Population PK analysis was performed using NONMEM®.
Results
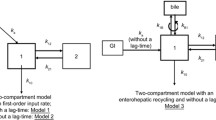
The PK of MPA and MPAG were best described by a five-chain compartment model, including a gallbladder compartment for EHC and a transit absorption model. Ethnicity was significantly correlated with the apparent clearance (CL/F) and volume of distribution (V/F) of MPAG but not those of MPA. Weight was identified as a covariate and was correlated with the PK of MPA and MPAG. MPA CL/F was 11.5 L/h for a 70-kg healthy subject, and the MPAG CL/F values were 1.36 and 1.90 L/h for 70-kg Chinese and Caucasian individuals, respectively. Internal and external evaluation indicated model validity.
Conclusions
This is the first population PK analysis to evaluate ethnic differences in the PK of MPA and MPAG in healthy Chinese and Caucasian subjects. No differences were observed in the PK of MPA between healthy Chinese and Caucasian subjects. Although, the MPAG CL/F was approximately 40 % higher in Caucasians, this finding may not be clinically relevant.



Similar content being viewed by others
References
USRDS (2013) Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States, (2013) National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. http://www.usrds.org/2013/pdf/v2_ch7_13.pdf. Accessed 7 Oct 2014
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47(2–3):85–118
Bullingham RE, Nicholls AJ, Kamm BR (1998) Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 34(6):429–455
Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P (2005) Identification of the UDP-glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos 33(1):139–146. doi:10.1124/dmd.104.001651
Staatz CE, Tett SE (2007) Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 46(1):13–58. doi:10.2165/00003088-200746010-00002
Cho EK, Han DJ, Kim SC, Burckart GJ, Venkataramanan R, Oh JM (2004) Pharmacokinetic study of mycophenolic acid in Korean kidney transplant patients. J Clin Pharmacol 44(7):743–750. doi:10.1177/0091270004266634
Jirasiritham S, Sumethkul V, Mavichak V, Na-Bangchang K (2004) The pharmacokinetics of mycophenolate mofetil in Thai kidney transplant recipients. Transplant Proc 36(7):2076–2078. doi:10.1016/j.transproceed.2004.08.087
Tsang WK, Tong KL, Yeung S, Lee W, Chan HW (2000) Efficacy and safety of mycophenolate mofetil in different dosages in Asian renal allograft recipients. Transplant Proc 32(7):1755–1756
Wang X, Tang X, Xu D (1998) Immunosupressive effect of mycophenolate mofetil with two different dosages in cadaveric renal transplantation:a short study. Transplant Proc 30(7):3573–3574
Jiao Z, Ding JJ, Shen J, Liang HQ, Zhong LJ, Wang Y, Zhong MK, Lu WY (2008) Population pharmacokinetic modelling for enterohepatic circulation of mycophenolic acid in healthy Chinese and the influence of polymorphisms in UGT1A9. Br J Clin Pharmacol 65(6):893–907. doi:10.1111/j.1365-2125.2008.03109.x
Jiang C, Chen H, Gui L, Pei Q (2009) Relative bioavailability and bioequivalence of mycophenolate mofetil in healthy volunteers. J Hunan Environ-Biol Polytech 15(4):13–17
Bullingham R, Monroe S, Nicholls A, Hale M (1996) Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol 36(4):315–324
Naderer OJ, Dupuis RE, Heinzen EL, Wiwattanawongsa K, Johnson MW, Smith PC (2005) The influence of norfloxacin and metronidazole on the disposition of mycophenolate mofetil. J Clin Pharmacol 45(2):219–226. doi:10.1177/0091270004271555
Geng F, Jiao Z, Dao YJ, Qiu XY, Ding JJ, Shi XJ, Li ZD, Zhong MK (2012) The association of the UGT1A8, SLCO1B3 and ABCC2/ABCG2 genetic polymorphisms with the pharmacokinetics of mycophenolic acid and its phenolic glucuronide metabolite in Chinese individuals. Clin Chim Acta Int J Clin Chem 413(7–8):683–690. doi:10.1016/j.cca.2011.12.003
Jiao Z, Zhong JY, Zhang M, Shi XJ, Yu YQ, Lu WY (2007) Total and free mycophenolic acid and its 7-O-glucuronide metabolite in Chinese adult renal transplant patients: pharmacokinetics and application of limited sampling strategies. Eur J Clin Pharmacol 63(1):27–37. doi:10.1007/s00228-006-0215-y
Liang MZ, Lu YP, Nan F, Li YP (2006) Pharmacokinetics of mycophenolic acid and its glucuronide after a single and multiple oral dose of mycophenolate mofetil in Chinese renal transplantation recipients. Transplant Proc 38(7):2044–2047. doi:10.1016/j.transproceed.2006.06.027
Shaw LM, Korecka M, Aradhye S, Grossman R, Bayer L, Innes C, Cucciara A, Barker C, Naji A, Nicholls A, Brayman K (2000) Mycophenolic acid area under the curve values in African American and Caucasian renal transplant patients are comparable. J Clin Pharmacol 40(6):624–633
Johnson AG, Rigby RJ, Taylor PJ, Jones CE, Allen J, Franzen K, Falk MC, Nicol D (1999) The kinetics of mycophenolic acid and its glucuronide metabolite in adult kidney transplant recipients. Clin Pharmacol Ther 66(5):492–500. doi:10.1016/s0009-9236(99)70012-3
Weber LT, Shipkova M, Lamersdorf T, Niedmann PD, Wiesel M, Mandelbaum A, Zimmerhackl LB, Schutz E, Mehls O, Oellerich M, Armstrong VW, Tonshoff B (1998) Pharmacokinetics of mycophenolic acid (MPA) and determinants of MPA free fraction in pediatric and adult renal transplant recipients. German study group on mycophenolate mofetil therapy in pediatric renal transplant recipients. J Am Soc Nephrol 9(8):1511–1520
Shen B, Li S, Zhang Y, Yuan X, Fan Y, Liu Z, Hu Q, Yu C (2009) Determination of total, free and saliva mycophenolic acid with a LC-MS/MS method: application to pharmacokinetic study in healthy volunteers and renal transplant patients. J Pharm Biomed Anal 50(3):515–521. doi:10.1016/j.jpba.2009.05.030
Xia DY, Gao FR, Qin HX, Yan M (2008) Pharmacokinetics of mycophenolate mofetil in Chinese healthy volunteers. Pharm J Chin PLA 24(3):218–220
Shipkova M, Armstrong VW, Wieland E, Niedmann PD, Schutz E, Brenner-Weiss G, Voihsel M, Braun F, Oellerich M (1999) Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Br J Pharmacol 126(5):1075–1082. doi:10.1038/sj.bjp.0702399
BIC selection procedures in mixed effects models (2012) INRIA. http://hal.inria.fr/docs/00/69/64/35/PDF/RR-7948.pdf. Accessed 7 Oct 2014
Vaida F, Blanchard S (2005) Conditional Akaike information for mixed-effects models. Biometrika 92(2):351–370. doi:10.1093/biomet/92.2.351
Byon W, Smith MK, Chan P, Tortorici MA, Riley S, Dai H, Dong J, Ruiz-Garcia A, Sweeney K, Cronenberger C (2013) Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometrics Syst Pharmacol 2:e51. doi:10.1038/psp.2013.26
Roberts MS, Magnusson BM, Burczynski FJ, Weiss M (2002) Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet 41(10):751–790. doi:10.2165/00003088-200241100-00005
Sherwin CM, Fukuda T, Brunner HI, Goebel J, Vinks AA (2011) The evolution of population pharmacokinetic models to describe the enterohepatic recycling of mycophenolic acid in solid organ transplantation and autoimmune disease. Clin Pharmacokinet 50(1):1–24. doi:10.2165/11536640-000000000-00000
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34(5):711–726. doi:10.1007/s10928-007-9066-0
Laporte-Simitsidis S, Girard P, Mismetti P, Chabaud S, Decousus H, Boissel JP (2000) Inter-study variability in population pharmacokinetic meta-analysis: when and how to estimate it? J Pharm Sci 89(2):155–167. doi:10.1002/(SICI)1520-6017(200002)89:2<155::AID-JPS3>3.0.CO;2-2
Wakefield J, Rahman N (2000) The combination of population pharmacokinetic studies. Biometrics 56(1):263–270
Boeckmann AJ, Sheiner LB, Beal SL (2013) NONMEM user’s guides. Icon Development Solutions Ellicott City, MD
Pitsiu M, Hussein Z, Majid O, Aarons L, de Longueville M, Stockis A (2004) Retrospective population pharmacokinetic analysis of cetirizine in children aged 6 months to 12 years. Br J Clin Pharmacol 57(4):402–411. doi:10.1046/j.1365-2125.2003.02017.x
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi:10.1146/annurev.pharmtox.48.113006.094708
Efron B, Gong G (1983) A leisurely look at the bootstrap, the jackknife and cross-validation. Am Stat 37(1):36–48
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9(4):503–512
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28(2):171–192
Sherwin CM, Sagcal-Gironella AC, Fukuda T, Brunner HI, Vinks AA (2012) Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with childhood-onset systemic lupus erythematosus. Br J Clin Pharmacol 73(5):727–740. doi:10.1111/j.1365-2125.2011.04140.x
Cremers S, Schoemaker R, Scholten E, den Hartigh J, Konig-Quartel J, van Kan E, Paul L, de Fijter J (2005) Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling. Br J Clin Pharmacol 60(3):249–256. doi:10.1111/j.1365-2125.2005.02398.x
Girard H, Court MH, Bernard O, Fortier LC, Villeneuve L, Hao Q, Greenblatt DJ, von Moltke LL, Perussed L, Guillemette C (2004) Identification of common polymorphisms in the promoter of the UGT1A9 gene: evidence that UGT1A9 protein and activity levels are strongly genetically controlled in the liver. Pharmacogenetics 14(8):501–515
Kuypers DR, Naesens M, Vermeire S, Vanrenterghem Y (2005) The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther 78(4):351–361. doi:10.1016/j.clpt.2005.06.007
Levesque E, Delage R, Benoit-Biancamano MO, Caron P, Bernard O, Couture F, Guillemette C (2007) The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther 81(3):392–400. doi:10.1038/sj.clpt.6100073
Sanchez-Fructuoso AI, Maestro ML, Calvo N, Viudarreta M, Perez-Flores I, Veganzone S, De la Orden V, Ortega D, Arroyo M, Barrientos A (2009) The prevalence of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T and its influence on mycophenolic acid pharmacokinetics in stable renal transplant patients. Transplant Proc 41(6):2313–2316. doi:10.1016/j.transproceed.2009.06.038
van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Schmidt J, Budde K, Kuypers D, Le Meur Y, van der Werf M, Mamelok R, van Gelder T (2009) UGT1A9–275T>A/-2152C>T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther 86(3):319–327. doi:10.1038/clpt.2009.83
Innocenti F, Liu W, Chen P, Desai AA, Das S, Ratain MJ (2005) Haplotypes of variants in the UDP-glucuronosyltransferase1A9 and 1A1 genes. Pharmacogenet Genomics 15(5):295–301
Saeki M, Saito Y, Jinno H, Sai K, Ozawa S, Kurose K, Kaniwa N, Komamura K, Kotake T, Morishita H, Kamakura S, Kitakaze M, Tomoike H, Shirao K, Tamura T, Yamamoto N, Kunitoh H, Hamaguchi T, Yoshida T, Kubota K, Ohtsu A, Muto M, Minami H, Saijo N, Kamatani N, Sawada JI (2006) Haplotype structures of the UGT1A gene complex in a Japanese population. Pharmacogenomics J 6(1):63–75. doi:10.1038/sj.tpj.6500335
Acknowledgments
The authors sincerely thank Dr. Bing Shen of the First Shanghai People’s Hospital (Shanghai, China) and Dr. Qi Pei of the Third Xiangya Hospital (Changsha, China) for providing their original pharmacokinetic datasets. The authors would also like to thank Dr. Nicolas Frey and Dr. Yuyan Jin of Roche, Ltd. for their invaluable comments. The help of Mr. Lixuan Qian of the Pharmacy School of Fudan University in preparing the manuscript is also appreciated. This project was partially supported by the National Natural Science Foundation of China (No. 81072702) and the Major Research and Development Project of Innovative Drugs, China Ministry of Science and Technology (2012ZX09303004-001).
Conflict of interest
Dr. Jun Shi and Dr. Qiudi Jiang are employees of the Roche Innovation Center Shanghai, China
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 281 kb)
Rights and permissions
About this article
Cite this article
Ling, J., Shi, J., Jiang, Q. et al. Population pharmacokinetics of mycophenolic acid and its main glucuronide metabolite: a comparison between healthy Chinese and Caucasian subjects receiving mycophenolate mofetil. Eur J Clin Pharmacol 71, 95–106 (2015). https://doi.org/10.1007/s00228-014-1771-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1771-1