Abstract
Purpose
Omeprazole has (R)- and (S)-enantiomers, which exhibit different pharmacokinetics (PK) among patients with cytochrome P450 (CYP) 2C19 genotype groups. The aim of this study was to investigate whether the 1-point, 4-h postdose (R)-omeprazole hydroxylation index (HI) of racemic omeprazole reflects the three CYP2C19 genotype groups in Japanese individuals.
Methods
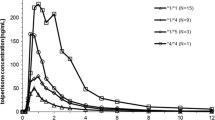
Ninety healthy Japanese individuals were enrolled and classified into the three different CYP2C19 genotype groups: homozygous extensive metabolizers (hmEMs; n = 34), heterozygous EMs (htEMs; n = 44), and poor metabolizers (PMs; n = 12). Blood samples were drawn 4 h after the intake of an oral dose of omeprazole 40 mg, and plasma levels of omeprazole and its metabolites were analyzed by high-performance liquid chromatography (HPLC) using a chiral column.
Results
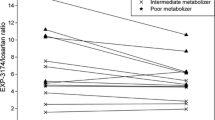
Mean plasma concentrations of (R)- and (S)-omeprazole in PMs were significantly higher than those in hmEMs and htEMs, and similar results were obtained in the case of omeprazole sulfone. Additionally, there was a significant difference in plasma concentrations of (R)-5-hydroxyomeprazole among CYP2C19 genotype groups, whereas no significant differences were observed in that of (S)-5-hydroxyomeprazole. Similarly, (R)-omeprazole HI in hmEMs, htEMs, and PMs were 5.6, 3.1, and 0.3, respectively, which were significantly different, but no significant difference was present in the (S)-omeprazole HI.
Conclusion
Our findings demonstrate that (R)-omeprazole HI correlated better with CYP2C19 genotype groups than racemic-omeprazole HI, and these results may be useful for classification among patients in CYP2C19 genotype groups prior to omeprazole treatment.

Similar content being viewed by others
References
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–598
Andersson T, Weidolf L (2008) Stereoselective disposition of proton pump inhibitors. Clin Drug Investig 28:263–279
Andersson T, Hassan-Alin M, Hasselgren G, Röhss K, Weidolf L (2001) Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet 40:411–426
Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH (2006) The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta- analysis. Am J Gastroenterol 101:1467–1475
Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kuriyama S, Iwaizumi M, Yamade M, Terakawa I, Ohashi K, Ishizaki T, Hishida A (2007) Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther 81:521–528
Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E (2001) Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther 69:158–168
Schwab M, Schaeffeler E, Klotz U, Treiber G (2004) CYP2C19 polymorphism is a major predictor of treatment failure in white patients by use of lansoprazole-based quadruple therapy for eradication of Helicobacter pylori. Clin Pharmacol Ther 76:201–209
Olbe L, Carlsson E, Lindberg P (2003) A proton-pump inhibitor expedition: The case histories of omeprazole and esomeprazole. Nat Rev Drug Discov 2:132–139
Mohammad M, Amir HDJ, Ahmad S, Neda M, Farinaz T (2010) A randomized controlled trial: Efficacy and safety of azathromycin, ofloxacin, bismutu and omeprazole compared with amoxicillin, clarithromycin, bismuth, and omeprazole as second-line therapy in patients with Helicobacter pylori infection. Helicobacter 15:154–159
Ogawa R, Echizen H (2010) Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet 1:509–533
Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T (1993) Oxidative metabolism of omeprazole in human liver microsomes: Cosegregation with S-mephenytoin 4′-hydroxylation. J Pharmacol Exp Ther 266:52–59
Andersson T, Miners JO, Veronese ME, Birkett DJ (1994) Identification of human liver cytochrome P450 isoform mediating secondary omeprazole metabolism. Br J Clin Pharmacol 35:597–604
Äbelö A, Andersson T, Antonsson M, Naudot AK, Skanberg I, Weidolf L (2000) Stereoselective metabolism of omeprazole by human cytochrome P450 enzyme. Drug Metab Dispos 28:966–972
Uno T, Niioka T, Hayakari M, Yasui-Furukori N, Sugawara K, Tateishi T (2007) Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol 63:143–149
Shimizu M, Uno T, Niioka T, Yasui-Furukori N, Takahata T, Sugawara K, Tateishi T (2006) Sensitive determination of omeprazole and its two main metabolites in human plasma by column-switching high-performance liquid chromatography: application to pharmacokinetic study in relation to CYP2C19 genotypes. J Chromatogr B Analyt Technol Biomed Life Sci 832:241–248
Ieiri I, Kubota T, Urae A, Kimura M, Wada Y, Mamiya K, Yoshioka S, Irie S, Amamoto T, Nakamura K, Nakano S, Higuchi S (1996) Pharmacokinetics of omeprazole (a substrate of CYP2C19) and comparison with two mutant alleles, C gamma P2C19m1 in exon 5 and C gamma P2C19m2 in exon 4, in Japanese subjects. Clin Pharmacol Ther 59:647–653
Kimura M, Ieiri I, Wada Y, Mamiya K, Urae A, Iimori E, Sakai T, Otsubo K, Higuchi S (1999) Reliability of the omeprazole hydroxylation index for CYP2C19 phenotyping: possible effect of age, liver disease and length of therapy. Br J Clin Pharmacol 47:115–119
Niioka T, Uno T, Sugimoto K, Sugawara K, Hayakari M, Tateishi T (2007) Estimation of CYP2C19 activity by the omeprazole hydroxylation index at a single point in time after intravenous and oral administration. Eur J Clin Pharmacol 63:1031–1038
Rosemary J, Adithan C, Padmaja N, Shashindran CH, Gerard N, Krishnamoorthy R (2005) The effect of the CYP2C19 genotype on the hydroxylation index of omeprazole in South Indians. Eur J Clin Pharmacol 61:19–23
Yin OQ, Tomlinson B, Chow AH, Waye MM, Chow MS (2004) Omeprazole as a CYP2C19 marker in Chinese subjects: assessment of its gene-dose effect and intrasubject variability. J Clin Pharmacol 44:582–589
Shiohira H, Yasui-Furukori N, Yamada S, Tateishi T, Akamine Y, Uno T (2012) Hydroxylation of R(+)- and S(−)-Omeprazole after Racemic Dosing are Different among the CYP2C19 Genotypes. Pharm Res 29:2310–2316
Zhou HH, Anthony LB, Wood AJ, Wilkinson GR (1990) Induction of polymorphic 4′-hydroxylation of S-mephenytoin by rifampicin. Br J Clin Pharmacol 30:471–475
Feng HJ, Huang SL, Wang W, Zhou HH (1998) The induction effect of rifampicin on activity of mephenytoin 4′-hydroxylase related to M1 mutation of CYP2C19 and gene dose. Br J Clin Pharmacol 45:27–29
Zhou HH (2001) CYP2C19 genotype determines enzyme activity and inducibility of S-mephenytoin hydroxylase. Clin Chim Acta 313:203–208
Wedlund PJ, Aslanian WS, Jacqz E, McAllister CB, Branch RA, Wilkinson GR (1985) Phenotypic differences in mephenytoin pharmacokinetics in normal subjects. J Pharmacol Exp Ther 234:662–669
Sugimoto K, Uno T, Yamazaki H, Tateishi T (2008) Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br J Clin Pharmacol 65:437–439
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Shiohira H, Yasui-Furukori N, Tateishi T, Uno T (2011) Chiral assay of omeprazole and metabolites and its application to a pharmacokinetics related to CYP2C19 genotypes. J Chromatogr B Analyt Technol Biomed Life Sci 879:2465–2470
Tybring G, Böttiger Y, Widén J, Bertilsson L (1997) Enantioselective hydroxylation of omeprazole catalyzed by CYP2C19 in Swedish white subjects. Clin Pharmacol Ther 62:129–137
Yasui-Furukori N, Takahata T, Nakagami T, Yoshiya G, Inoue Y, Kaneko S, Tateishi T (2004) Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes. Br J Clin Pharmacol 57:487–494
Li X, Weidolf L, Simonsson R, Andersson TB (2005) Enantiomer/Enantiomer interaction between the S- and R-isomers of omeprazole in human cytochrome P450 enzymes: major role of CYP2C19 and CYP3A4. J Pharmacol Exp Ther 315:777–787
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (No. 20590150) Tokyo, Japan.
Conflict of interest statement
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamada, S., Shiohira, H., Yasui-Furukori, N. et al. The (R)-omeprazole hydroxylation index reflects CYP2C19 activity in healthy Japanese volunteers. Eur J Clin Pharmacol 69, 1423–1428 (2013). https://doi.org/10.1007/s00228-013-1480-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1480-1